This new approach allows us to understand how human nerve cells connect to muscle. These connections are essential for our bodies to be able to move. By growing these connections using human stem cells we hope to better understand how things go wrong in diseases like motor neuron disease or muscular dystrophy. This platform lets us test potential drugs without having to use animals."
Dr Peter Harley, Lead author
06 March 2023
Microdevices to grow human neurons and muscle could accelerate the treatment of diseases like ALS
King’s researchers have developed a microdevice which mimics how muscle cells and neurons grow in the human body, allowing them to test preventative drugs for neuromuscular diseases

Amyotrophic Lateral Sclerosis (ALS), sometimes known as Lou Gehrig’s Disease, is caused by a progressive and rapid degeneration and eventual death of the neurons that control movement. Most people with Amyotrophic Lateral Sclerosis (ALS) will die from respiratory failure 3 to 5 years after the onset of symptoms. Yet, despite decades of research, there are still no effective treatments which can halt the progression of the condition. Progress in treating neuromuscular diseases has been hindered by a lack of understanding about what exactly goes wrong with the muscle and neurons.
A growing body of research has highlighted that dysfunction at the interface, or synapse, between motor neurons and muscle is heavily implicated in the cause and progression of neuromuscular diseases. Many researchers are now interested in studying neuromuscular synapses and targeting them in future treatments, but this requires a model which accurately represents human neuromuscular physiology (as opposed to mouse models). A promising alternative is to grow muscle cells and neurons from patient stem cells in a dish, but it has been challenging to do this in a way which represents the complex organization of the human body.
A new technique from researchers the Centre for Gene Therapy & Regenerative Medicine and Centre for Developmental Neurobiology at King’s College London, in collaboration with colleagues at Queen Mary University London and National University of Singapore, overcomes this challenge with a tiny 3-dimensional device, just 2.5mm long, which mimics the separation between central nervous system and other parts of the body, such as muscle. The microdevice has 3 chambers which muscle cells and neurons can be grown in separately whilst still allowing neurons to grow projects and innervate the muscle via connecting channels which are 100x smaller than a millimeter. This unique design prevents muscle cells from dethatching when they contract which allows cells to mature and the cells to be studied over longer culture periods.
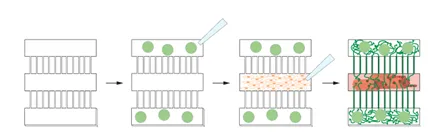
Demonstrating the promise of the device, the team grew two distinct populations of neurons in separate chambers – one which has been genetically altered to send signals following light situation and one which does not – but still innervating the same muscle target. High powered imaging allowed detailed observation of neuromuscular synapse number and shape and showed the light stimulated neurons had much greater synapse formation and muscle fiber contraction. Future studies using this simple but effective model could use patient-derived neurons and muscle cells to test candidate drugs that prevent or slow muscle deterioration.
Read the full paper 3D Compartmentalised Human Pluripotent Stem Cell–derived Neuromuscular Co-cultures.

