“We are really pleased to have been able to support the development of the MR MinMo device. We have worked closely with the research team in the design process to provide instruction and templates that facilitated the generation of compliant technical documentation for submission to the MHRA. This is a great example to other research groups aiming for regulatory approval.”
Clare Heaysman, Senior Lecturer in MedTech Regulatory Affairs and Director of Medical Engineering Quality Management System.
15 July 2025
New head stabilisation device aims to improve MRI image quality
A device developed by researchers at the School of Biomedical Engineering & Imaging Sciences that aims to keep the head more stable in brain MRIs has been given the go-ahead to be tested in the clinic.
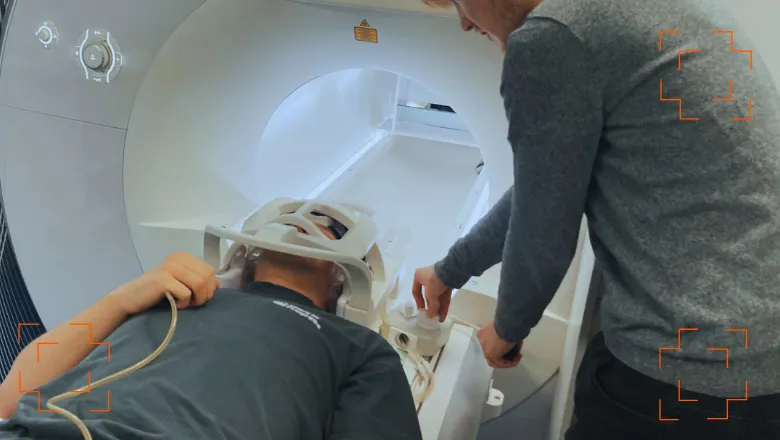
Each year in England, the NHS performs nearly one million brain scans using magnetic resonance imaging (MRI). These scans require patients to lie still for 2-10 minutes at a time, and up to 60 minutes in total. Even slight movements can result in inaccurate images and may mean the scan needs to be repeated. Maintaining this level of stillness is difficult for anyone, but can be particularly challenging for young people or individuals with brain injuries or neurological diseases.
To reduce this problem, a research team led by Professor David Carmichael at King’s College London, have developed the Magnetic Resonance Minimal Motion (MR MinMo), a device that aims to keep the head more stable during MRI by combining a static frame with adjustable yet firmly fixed pads and inflatables.
The team, which includes colleagues from University College London and A94 Technologies, aim to make their research device into a manufacturable product that can be clinically validated. They are now a step closer to this, as they recently received approval from the Medicines and Healthcare products Regulatory Agency (MHRA) to test their device in the clinic.
The team worked with the Medical Engineering Quality and Regulatory Team within the School, which offers regulatory support for medical device development. This project is the first to get MHRA approval for clinical investigation following the Quality Management System (QMS) processes.
Over the coming months, the team will compare their device to standard practice in children undergoing head MRI for clinical purposes at the Evelina hospital, hoping to show it can increase scan quality and reduce the number of repeated scans, saving money for the NHS.
“This is a really exciting moment in the MR MinMo development because we can start to test its efficacy in a real clinical environment and understand its impact on improving image quality and reducing costly scan failures.”
David Carmichael, Professor in Magnetic Resonance Imaging.


