A child born with HLHS presents a unique anatomy and each clinical decision needs to be tailored to it. This is very challenging. In this context, our key objective is to deliver non-invasive technology that assesses the health and function of our main vessels. With such technology, surgical processes can be tailored to alleviate existing problems or can be evaluated and further improved by studying the impact of previous procedural choices.
Dr Adelaide De Vecchi, Lecturer, School of Biomedical Engineering & Imaging Sciences
04 March 2022
New MRI method will better inform surgical decisions for babies born with a heart defect
A novel 10-minute MRI scan could help surgeons better plan for a congenital heart defect
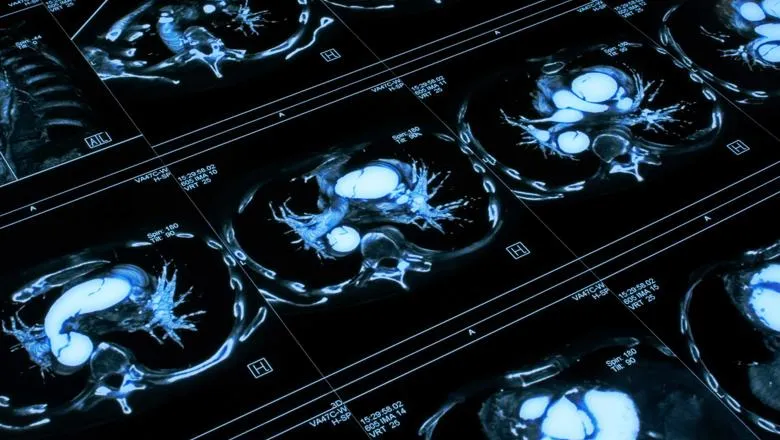
Researchers from the School of Biomedical Engineering & Imaging Sciences have identified a novel method of tracking the health of the aorta to better inform surgical decisions and patient monitoring, particularly for congenital conditions such as hypoplastic left heart syndrome (HLHS).
Published in the Journal of Cardiovascular Translational Research, the research focuses on the aorta, the main artery that receives the blood of each heartbeat and distributes it to our body.
The large arteries, particularly the aorta, serve as a conduit to transport the blood from the ventricles to the body, and as a reservoir of blood that fills during systole (period of contraction of the ventricles of the heart that occurs between the first and second heart sounds of the cardiac cycle) and recoils during diastole (the phase of the heartbeat when the heart muscle relaxes and allows the chambers to fill with blood).
The researchers developed a non-invasive comprehensive way to study these two functions along the aorta from a 10-minute MRI scan, replacing the need of invasive catheterised methods that were not even close to report the rich spatial information the new method can provide.
HLHS is a birth defect where babies are born with an under-developed left part of the heart. Currently, immediately after birth, babies require staged surgical palliation – a series of procedures that save the life of the babies and that aim to maximise life expectancy and relieving complications.
As part of these procedures, surgeons also need to enlarge the aorta so that it can accommodate the entire cardiac output.
Despite numerous patients now surviving the staged palliation procedure, they live with only half of their heart – a single ventricle instead of two – and are thus more likely to have cardiac complications over time.
It is paramount to minimise the presence of unfavourable blood flow conditions, such as those caused by sub-optimal surgical reconstructions of the aorta.
There is also increasing evidence that significant neurodevelopmental issues associated with HLHS may be triggered by these cardiac factors.
With our novel method, we now have the ability to evaluate with unprecedented detail the blood flow pathophysiology of the aorta artery through the conduit and reservoir functions.
Dr Alessandro Faraci, Research Associate, School of Biomedical Engineering & Imaging Sciences
In the future this will even allow us to guide the definition of optimal strategies for surgical and clinical treatment.
Mr Joao Filipe Fernandes, Research Student, School of Biomedical Engineering & Imaging Sciences
This work was supported by Action Medical Research and Great Ormond Street Hospital Children’s Charity.


