Revealing that nearly half of the mutated genes causing neuromuscular disorders could directly alter satellite cells - hindering muscle repair - these studies finally highlight a common mechanism across different conditions, also setting a milestone for future research and the development of tailored interventions.”
Dr Massimo Ganassi
29 March 2022
New studies highlight a common mechanism for declining muscle function
The results improve our understanding of neuromuscular disorders
Neuromuscular disorders can be caused by hereditary or acquired genetic mutations, impairing the function of skeletal muscles: those under voluntary control. These diseases cause muscles to weaken and waste progressively, leading to declining muscle function and significant hinderance to quality of life.
Due their diverse origins and clinical presentations, neuromuscular disorders have remained challenging to assess and scientists have struggled to identify common pathological mechanisms.
In two recent studies Dr Massimo Ganassi and Professor Peter Zammit, both based in the School of Basic & Medical Biosciences, have investigated how genetic mutations affect muscle stem cells while they regenerate muscle fibres, resulting in muscle damage that is inefficiently repaired.
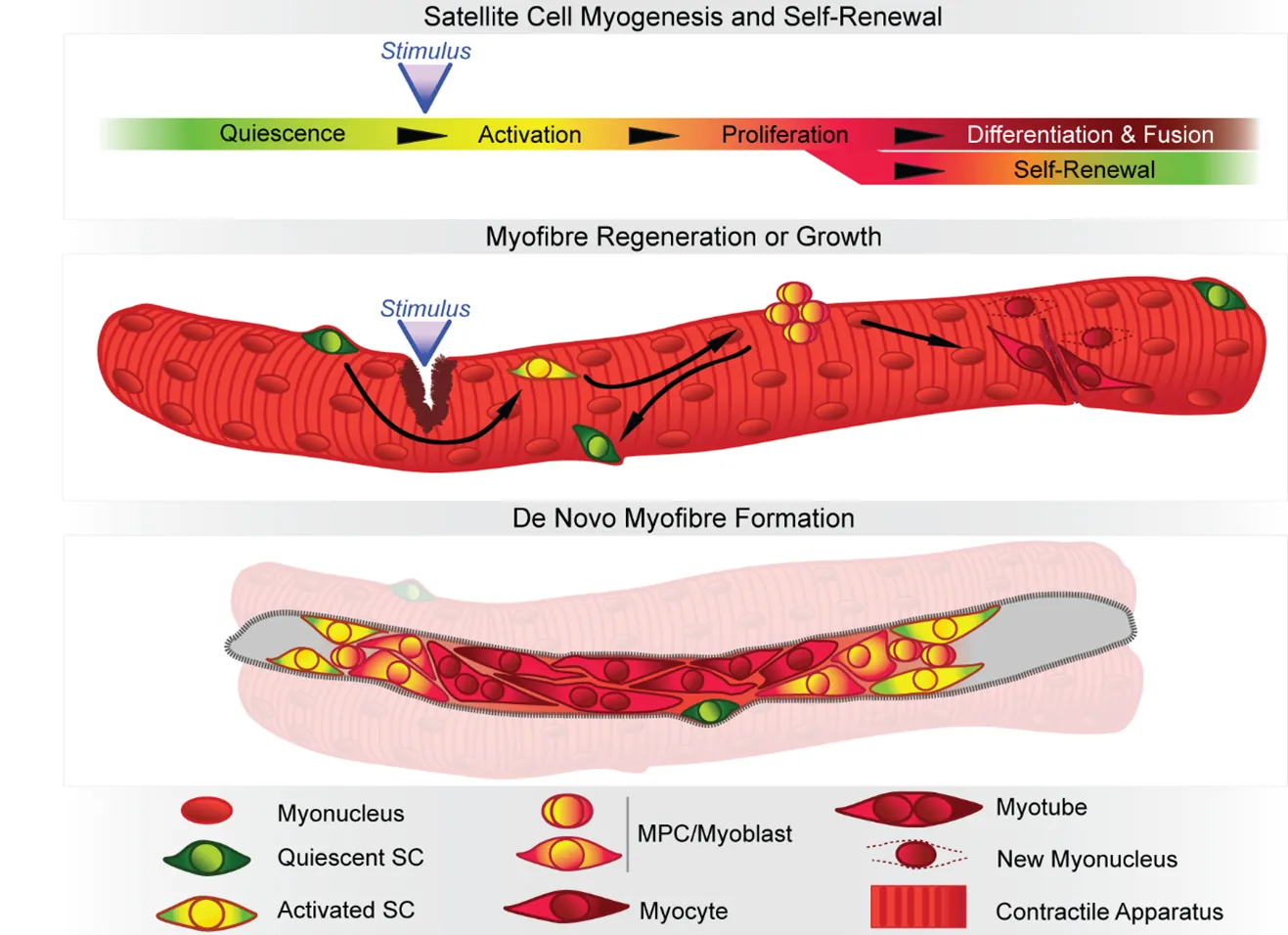
When healthy, skeletal muscles have great ability to self-repair and regenerate their muscle fibres if damaged after intense usage or trauma. Their resilience relies mostly on the resident population of stem cells, named satellite cells, which activate, proliferate, differentiate and fuse to regenerate muscle fibres after injury.
But mutations affecting genes functioning in any of the steps undertaken by the satellite cells to repair muscle fibres are likely to blunt regeneration, impeding life-time muscle turnover and leading to progressive weakness and wasting commonly observed in neuromuscular disorders.
In the original study, published in Experimental Cell Research this February, the authors considered congenital muscular dystrophies and myopathies, archetypical conditions where wasted muscle does not regenerate well, suggesting defective satellite cell performance. They were able to define a new class of neuromuscular disorders, the Satellite Cell-opathies - diseases where pathogenic mutations compromise the function of genes directly involved in satellite cell function.
In a follow-up study, published in European Journal of Translational Myology this March, they highlighted that 45% of genes associated with all neuromuscular disorders reported to date, including those with prominent involvement of nerves or heart, are also expressed in the satellite cells in their early phases of muscle repair, suggesting that dysfunction of any of these genes may hinder proper muscle regeneration. This outcome provides a mechanism that contributes to the declining muscle function and the compromised regeneration observed.
Driven by these findings the scientists will now investigate whether and how the function and status of satellite cells is affected in specific neuromuscular conditions. Their hope is that further characterization and classification of neuromuscular disorders based on the degree of involvement of satellite cells will result in development of prognostic and diagnostic tools and, ultimately, new therapeutics tailored to restore satellite cell function as part regenerative medicine based therapies.