Large-scale biomedical resources like the UK Biobank are paving the way for patient-centric disease characterisation by enabling detailed analysis of anatomical and electrophysiological variability across the population. This work demonstrated differences in cardiac axes among healthy and diseased individuals, highlighting the potential to enhance digital twin personalisation and improve disease prediction and characterisation, ultimately supporting more tailored clinical care.
Mohammad Kayyali, lead author and PhD student at the King's School of Biomedical Engineering & Imaging Sciences
10 July 2025
Researchers unlock hidden geometry of the heart to revolutionise ECG interpretation
A study led by scientists at King's has revealed how the physical orientation of the heart inside the chest dramatically influences the electrical signals captured in an electrocardiogram (ECG) - a discovery that could pave the way for more personalised and accurate heart diagnostics.
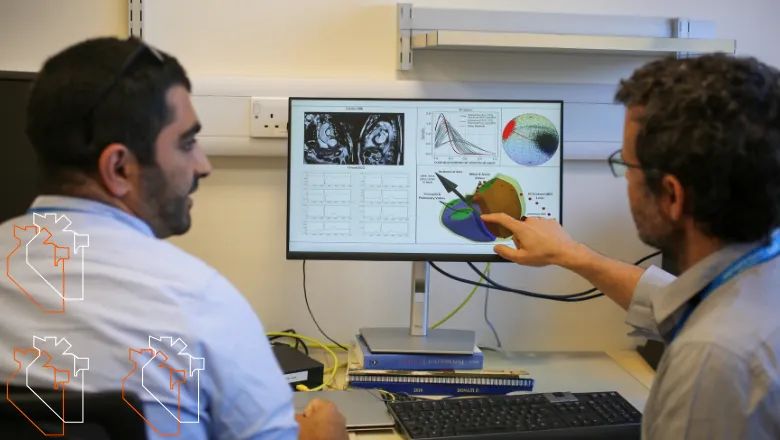
With data from over 39,000 participants from UK Biobank, this is one of the largest population-based studies to date exploring the relationship between heart anatomy and electrical activity. By combining 3D heart imaging with ECG data, the team created simplified digital twins of each participant’s heart. These personalised models allowed researchers to explore how the heart’s anatomical position, known as the anatomical axis, aligns with a spatial metric of electrical activity, or electrical axis.
Digital twins are emerging as a powerful tool in cardiovascular research, enabling scientists to simulate and study the heart’s structure and function in unprecedented detail. In this study, they were key to revealing how natural variations in heart orientation, shaped by factors such as body mass index (BMI), sex, and hypertension, can significantly influence ECG readings.
The researchers proposed new, standardised definitions for both anatomical and electrical axes based on their alignment in 3D space. They found that people with higher BMI or high blood pressure tend to have hearts that sit more horizontally in the chest, and that this shift is mirrored in their ECG signals. The study also revealed clear differences between men and women: male hearts were generally more horizontally oriented than female hearts, a structural difference that was reflected in their surface electrical activity. These sex-based variations highlight the need for more tailored approaches to ECG interpretation.
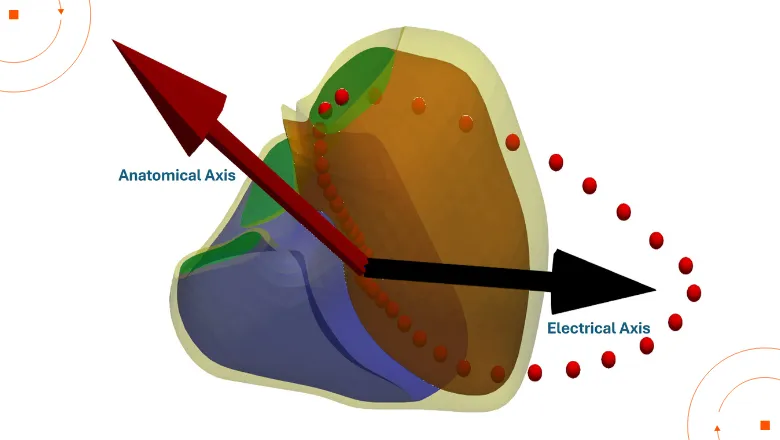
By identifying and quantifying this variability across a large population, the study highlights the importance of distinguishing between normal anatomical differences and early signs of disease. This could help clinicians detect conditions such as hypertension, conduction abnormalities, or early heart muscle changes sooner and more precisely - especially in patients whose heart orientation deviates from standard assumptions.
The ability to build personalised models (i.e. digital twins) of the cardiovascular system is an exciting research area, where we hope to find new parameters that can better inform about prevention, diagnosis and risks of cardiovascular diseases. In this work, we start to explore these uncharted waters, and we hope we will soon propose new ways to early detect conditions such as electrical conduction disorders.
Professor Pablo Lamata, report author and professor of biomedical engineering at the School
The findings point to a future where ECGs are no longer interpreted using a one-size-fits-all approach, but instead tailored to each patient’s unique anatomy. This personalised perspective could reduce misdiagnoses and support earlier, more targeted interventions.


