05 June 2019
Smart regulation of cell-matrix contacts
New study examines the mechanisms mediating the cross-talk between microtubules and two classes of integrin-based cell adhesions: focal adhesions and podosomes
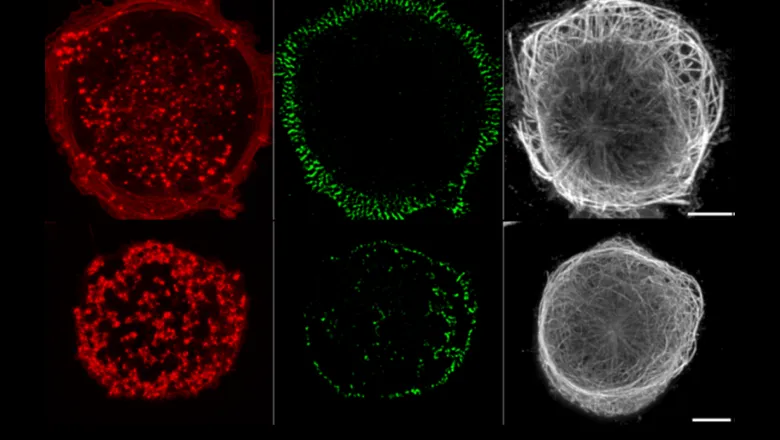
It has been known for many years that most tissue cells adhere to and often migrate within an extracellular matrix that they both make and remodel in which strong integrin-based adhesions (via focal adhesions) are required in order to generate strong anchorage and traction force. An exception to this generality is to be found in cells of monocytic lineage which migrate much more rapidly within connective tissues as part of their immune surveillance and phagocytic functions. In this case, strong focal adhesions would likely be a major hindrance to their rapid migratory behaviour and such cells have developed a second type of integrin-based adhesion system known as podosomes.
For some cells it is possible to see a switch between the stable focal adhesion system to that of a podosome; examples usually being highly invasive cancer (metastatic) cells. The generation of podosomes, or their near-equivalent invadosomes, is often regarded as a signature of an invasive phenotype with serious implications for cancer dissemination.
For the last few decades, a considerable effort has been made by many laboratories to discover how the switch between focal adhesions and podosomes is controlled, it is generally assumed that the two systems were differentially regulated since part of the cytoskeletal apparatus known as microtubules appear to have opposite effects on adhesion integrity. Importantly, experimental disruption of microtubules enhances focal adhesion formation and stability whilst the exact opposite is true of podosomes; they rapidly disassemble when microtubules are disrupted.
In a paper published in Nature Materials, Professor Gareth Jones, Randall Centre for Cell & Molecular Biophysics, and colleagues at the Mechanobiology Institute NUS, found a common mechanism underlying the effect of microtubules on both focal adhesions and podosomes. This unexpected outcome is mediated by the KANK family of adhesion-linking proteins and a RhoA activator GEF-H1 which governs myosin IIA filament production within a cell. Disruption of KANK-mediated links between microtubules and both types of contacts reproduces the effect of complete disruption of microtubules on these structures, without disruption of microtubules.
In previous publications, the authors found that when large numbers of myosin IIA filaments were induced, focal adhesion formation was promoted as was their associated bundles of contractile acto-myosin stress fibres. But how does microtubule disconnection affect myosin? Their new discovery describes how filaments are generated through a well-established signalling cascade whereby active GEF-H1 in the cytosol activates the small GTPase RhoA which in turn activates ROCK, a kinase that directly mediated myosin filament assembly. Inactive GEF-H1 is found attached to microtubules and is unable to be activated unless released. If microtubules are attached to integrins via a series of linking proteins including KANK, GEF-H1 is trapped on the microtubules and thus cannot drive myosin IIA filament production. In this case, a “default” adhesion system based on podosomes that are myosin IIA independent arises.
This simple system of control gives rise to a regulatory switch between the two major integrin-based adhesion systems in cells and whilst many questions still remain to be solved, the model provided by this paper gives the basis of future discovery paths that may impact on our ability to modulate the migratory behaviour of dangerously metastatic cancer cells.
