Support for patients and families living with genetic conditions
Academic research combines with clinical practice at King’s to support families affected by inherited genetic conditions.
10 December 2014
Our researchers developed diagnostic tests that led to the first child being born free of the genetic mutation which causes motor neurone disease.
Research led by Professors Chris Shaw, Ammar Al-Chalabi, Corinne Houart, Sarah Guthrie, Chris Miller & Drs Safa Al-Sarraj, Jean-Marc Gallo & Frank Hirth
Our researchers developed diagnostic tests that led to the first child being born free of the genetic mutation which causes motor neurone disease.
Every day, almost 400 people around the world are diagnosed with amyotrophic lateral sclerosis (ALS), the most common form of motor neurone disease. Our researchers have identified genes that, when mutated, cause some types of this progressive and fatal condition.
Their work has led to the development of genetic tests that identify mutations on genes named SOD1, FUS, TARDBP and C9orf72. These tests are now available in diagnostic laboratories around the world and can reveal whether someone carries a mutated version of the gene and therefore has an increased risk of developing ALS. They can also confirm diagnosis and inform treatment plans.
Our researchers have worked with Guy’s & St Thomas’ NHS Foundation Trust to translate discoveries into tests and procedures, including ‘pre-implantation genetic diagnosis’ (PGD) for ALS caused by a mutation on the SOD1 gene. ‘PGD gives people who carry a mutated gene the opportunity to avoid passing it on to their children,’ says Professor Chris Shaw, who led the research.
The technique involves genetic testing of an embryo created through in-vitro fertilisation where only embryos free from the genetic mutation are implanted in the womb. PDG for ALS due to a mutation on the SOD1 gene is now licensed by the Human Fertilisation Embryology Authority and was successfully used for the first time in the UK at Guy’s Hospital in 2013 when a baby boy was born free of the mutated gene that his mother carries. ‘The mutated version of the SOD1 gene had taken the lives of his grandmother and uncle,’ says Professor Shaw. ‘This was an amazing result for the family.’
In ALS, motor neurones in the brain and spinal cord degenerate causing the muscles they control to weaken and waste away. Symptoms normally start in mid-life and eventually affect all movement including swallowing and breathing. Average life expectancy from symptom onset is two to five years. Around 5,000 people in the UK have ALS at any time and 10 per cent of cases run in families.
Using sophisticated gene sequencing technology, and with the help of DNA donated by patients and their families, our researchers identified ALS-causing mutations in FUS, TARDBP and SOD1 genes and were the first to identify the location of C9orf72. This is the most common ‘ALS gene’ and causes 20 per cent of familial ALS and 10 per cent of sporadic cases.
‘Identifying genetic mutations that are linked to ALS improves diagnosis and means at-risk families can be screened and counselled,’ says Professor Shaw.
Discovering the genetic causes of inherited ALS can also help researchers understand more about the molecular mechanisms of the disease. ‘Although the population of people with the familial type of the disease is small, the biological impact of gene discoveries is huge. Genetic research gives us the strongest clues to the causes of the disease,’ he says.
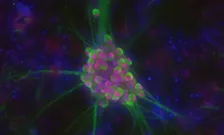
The team has also demonstrated, in ALS motor neurons (pictured above), that mutated TDP-43 protein accumulates inside motor neurones and initiates degeneration in 95 per cent of ALS cases.
‘Identifying genetic mutations will ultimately enable the development of new and effective treatments that can stop or ameliorate the symptoms of ALS,’ says Professor Shaw. ‘The more we understand about fundamental molecular mechanisms, the closer we are to developing effective therapies.’