
Biography
I investigate the biology of cells which become stuck, or “arrested”, during mitosis, and how cells that are arrested in mitosis drive cell death. Failure of mitotically arrested cells to die can lead to chromosomal instability, a hallmark of cancer, whilst anti-mitotic drugs used in the treatment of cancer can promote cell death by inducing mitotic arrests. Therefore a greater understanding of the underlying properties of mitotic arrests could provide more information of when best to use anti-mitotic drugs as chemotherapy, and lead to the development of novel drug targets. I investigate the biology of mitotic arrests and their role in driving cell death by utilising a wide array of techniques including: optogenetics, CRISPR, live and fixed cell imaging, and proteomics. I joined King’s College London as a Professor Anthony Mellows Medal/King’s Prize Fellow at the Randall Centre for Cell and Molecular Biophysics in 2022. Prior to joining King’s, I worked as a postdoctoral researcher investigating the mitotic Spindle Assembly Checkpoint and microtubule-kinetochore attachments in the lab of Prof Ulrike Gruneberg at the Sir William Dunn School of Pathology, University of Oxford, and lectured in Biochemistry at New College, University of Oxford. My PhD research, under the supervision of Prof James Wakefield at the University of Exeter, focused on the multiple synergistic methods of mitotic spindle assembly using Drosophila melanogaster embryos as a model system.
Research
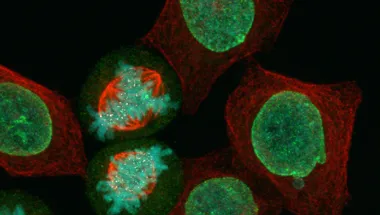
Hayward Group
We investigate the biology of cells that are arrested in mitosis, and how mitotic arrests drive cell death, utilising a wide array of techniques including: optogenetics, CRISPR, live and fixed cell imaging, and proteomics
Research

Hayward Group
We investigate the biology of cells that are arrested in mitosis, and how mitotic arrests drive cell death, utilising a wide array of techniques including: optogenetics, CRISPR, live and fixed cell imaging, and proteomics
