Correlative Light and Electron Microscopy (CLEM)
CLEM is an imaging technique that combines fluorescence light microscopy (FM) and electron microscopy (EM) to analyse the same biological sample. It merges the molecular specificity of FM with the high-resolution structural details of EM, allowing researchers to visualise, locate, and track specific molecules within their cellular context
CLEM is crucial for understanding the relationship between structure and function at a sub-cellular level.
Examples of use
Immuno-electron microscopy is powerful when employed to localise molecules within their functional intracellular locations. However, for rare events or those associated with a dynamic process, it is difficult to have confidence that the structure of interest can be found in a transmission electron microscope. To achieve this we need a specific area to be correlated across length and volume scales. In its most simple form CLEM allows a fluorescent protein, followed by live-cell imaging, to be tracked and processed for imaging by immuno-EM.
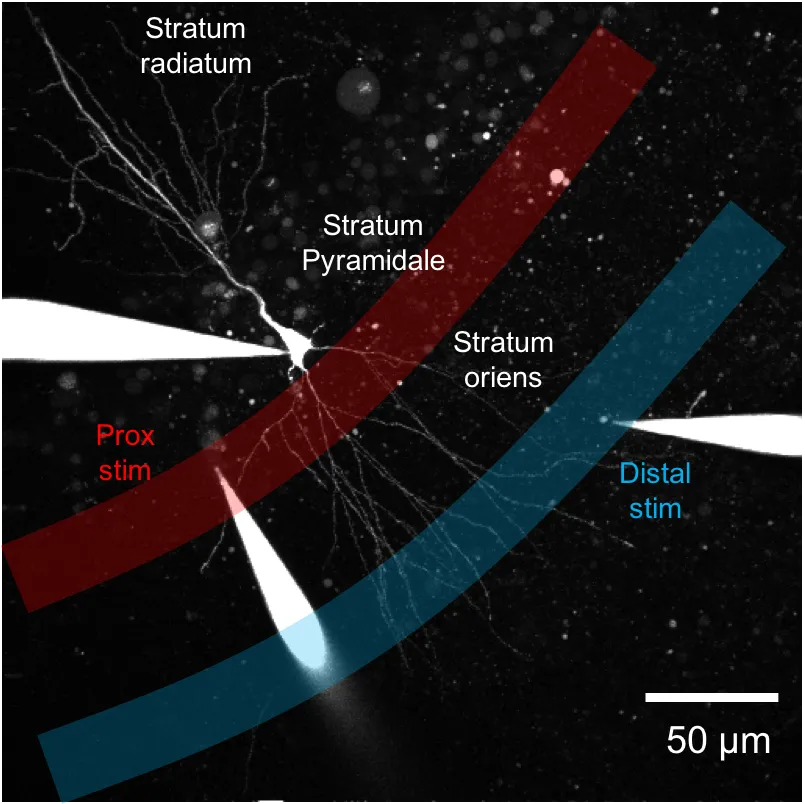
We also combine infrared branding in the Nikon Imaging Centre with serial block face scanning electron microscopy to correlate underlying ultrastructure in 3D with electrophysiology studies.
Equipment available
- JEOL JEM 1400Flash
- JEOL JEM F200.