High-Pressure Freezing and Freeze Substitution
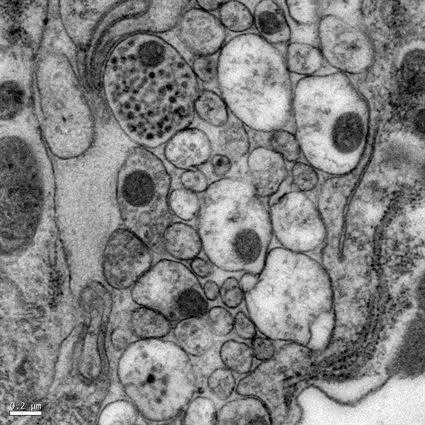
Many methods allow rapid freeze fixation/immobilisation of tissues. However, the depth of vitrification is often limited (eg a few microns for samples plunged into liquid cryogen).
Of the available technologies, high-pressure freezing (HPF) is by far the most effective.
HPF exploits the physical benefit of high pressure to reduce the cooling rate required for the vitrification of water making vitrification of tissues practicable. HPF machines synchronize pressurisation and cooling of the sample (to below the glass transition temperature (Tg)) within 20ms, extending the depth of vitrification to as much as 200μm.
Tissue samples prepared this way require modification to make them compatible with a transmission electron microscope. They must either be converted to a room-temperature stable state (eg by freeze substitution into a resin), or be sectioned and imaged below the Tg. When this is done, images close to the native structure of biological specimens can be achieved.
Freeze substitution equipment is also available at the centre.
HPF advantages:
- fast immobilisation with no loss of time resolution
- no loss of ions/molecules or lipids
- no depolymerization of proteins
- no denaturation of enzymes
- membranes maintain their permeability
- no osmotic effects
- preserves antigenicity
Examples of use
This sample preparation technique is specially recommended for specimens that are not amenable to chemical fixation due to the presence of a cuticle or thick cell walls such as yeast, C. elegans or plant samples.
The technique is also useful in cell biology studies with regular samples when we want to work with material closest to the living state.
Equipment available
- Leica-microsystems EM ICE

