MOBILE MEN
Overview
Providing Informed Choice for HIV Prevention for Men who are Mobile for work (MOBILE MEN) is a three-year (2023-2026) project funded by the European Commission’s EDCTP Horizon programme to help men who are mobile for work prevent HIV through scaling up their access to PrEP options.
MOBILE MEN works in South Africa and Uganda by implementing MALE-centred research to identify, understand, and remove barriers to ORAL AND INJECTABLE PrEP particularly in men who are mobile for work; coordinate with global, national, and subnational stakeholders to improve PrEP access and scale-up; and strengthen the capacity of local partners to support the delivery of HIV prevention products to men.
The MOBILE MEN consortium is led by Kings College along with core partners Desmond Tutu Health Foundation, Africa Health Research Institute, MRC/UVRI and LSHTM, London School of Hygiene & Tropical Medicine and University College London. Local partner co-leadership and engagement is central to the project's approach to improving men's access to HIV prevention products. Working closely with communities, MOBILE MEN will advance access to and uptake of PrEP that includes oral pre-exposure prophylaxis (oral PrEP) and injectable long-acting cabotegravir (CAB PrEP). Although the work takes place in Uganda and South Africa, we are linked in with other prevention programmes across sub-Saharan Africa.
The study is open and recruitment ended in November 2024.
Primary Objective
To assess implementation effectiveness of LA-CAB and oral FTC-TDF (daily and coitally dependent) on retention in care, coital coverage, and PrEP choice in men who are mobile for work
Secondary objectives:
- To describe adoption (acceptability, feasibility, attitudes towards and preferences) of on-demand and injectable PrEP
- To understand the reach of PrEP amongst mobile men and understand the barriers to uptake
- To understand patterns of use of daily, on-demand, and LA-CAB PrEP amongst different groups of mobile men and how choices/preferences change over time.
- To understand implementation of LA-CAB for men amongst service providers to inform scale-up
- To understand the appropriateness, feasibility and fidelity of delivering on-demand and long-acting PrEP in different settings
- To describe how on-demand and long-acting PrEP are delivered in practice
- To describe the service-level needs to implement on-demand and long-acting PrEP
- To evaluate antibody based monitoring HIV status for LA-CAB.
- To assess maintenance (affordability), cost and cost effectiveness of oral PrEP, Cab-LA and of simultaneous provision of both types of PrEP in Uganda and South Africa.
This project is funded by the European Commission, under the Global Health EDCTP3 Joint Undertaking work programme.
Methods
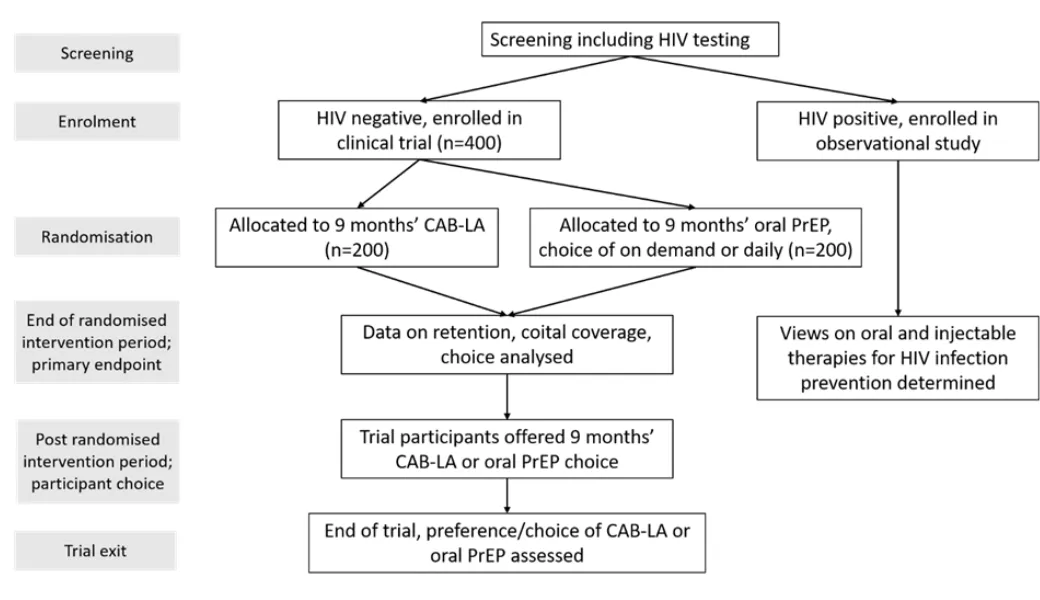
Open-label study of 400 men from mobile groups in South Africa and Uganda randomised to oral TDF-FTC or CAB-LA over 9 months, then access to both to 18 months. Service level process data and qualitative interviews (in depth and group discussions) with service users and service providers will run alongside the trial to evaluate preference, choice and implementation in real time. We evaluate for the first time, the implementation (including cost-effectiveness and modelling) of both LA-CAB and on demand PrEP for vulnerable men in Africa. This data gap in men for both types of PrEP will inform the WHO HIV prevention modelling and allow men in Africa access to on demand PrREP and LA-CAB.
Key updates:
- Recruitment ended November 2024: 400 HIV uninfected men randomised to either CAB-LA injection or oral TDF/FTC PrEP.
- Baseline survey confirms high mobility of men and their high-risk behaviours.
- 99% uptake of PrEP at enrolment.
- PrEP Choice phase begins from month 9, with the first month 9 visit due April 2025.
- Last participant visit expected March 2026.
Researchers throughout Europe have access open to them under the Mobile Men Grant Agreement. The publication policy document is under development by UVRI.
Our Partners

Desmond Tutu Health Foundation

MRC/UVRI and LSHTM Uganda Research Unit

Assistance Publique – Hôpitaux de Paris

University College London

HE2RO

Africa Health Research Institute

European Commission

Principal Investigator
Affiliations
Funding
Funding Body: European Commission
Amount: € 4,665,192.50
Period: July 2023 - July 2026



