03 October 2018
Adult stem cells control their own fate
A team of researchers at King’s College London and their collaborators have discovered why laboratory-grown tissues may fail when used to treat a range of conditions. For the last 20 years stem cells have been routinely placed within 3D biodegradable materials or “scaffolds” to grow new tissues to treat conditions such as osteoarthritis or heart failure. The discovery of why these engineered tissues have often failed to live up to their promise may now resolve some long-standing conflicts in the field and enable more successful transplants in the future.
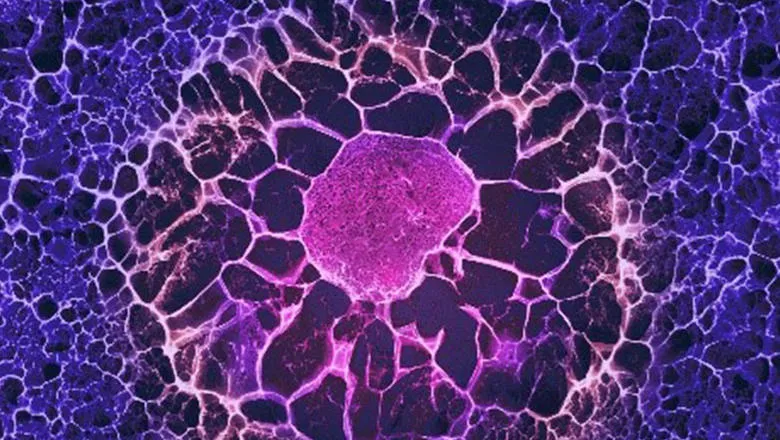
When used for tissue engineering, scaffolds contain instructions to direct the stem cells inside how to differentiate. However the study led by Dr Eileen Gentleman from King’s College London found that stem cells quickly modify the 3D scaffold they are placed within, changing both its composition and stiffness, rendering the instructive cues ineffective. Stem cells then rely on this new self-assembled matrix to direct their own differentiation, rather than cues from the scaffold they were originally placed within.
The study is a collaboration by a team of researchers at King’s College London, Imperial College London, University College London and the Francis Crick Institute. The impact for the field will be that scaffold design will no longer be simple. Researchers will now have to design scaffolds that take into account that cells will modify their surroundings once they are inside.
The paper, published in Nature Communications this week, shows that human stem cells derived from the bone marrow respond to being placed in 3D scaffolds by either secreting proteins around themselves, creating a stiff nest-like structure, or by releasing factors that degrade and soften their surroundings, and that these modifications themselves instruct the stem cells how to differentiate. The team’s findings suggest that researchers will have to re-think how best to design 3D scaffolds to create replacement tissues, as scaffold themselves may not directly instruct stem cells how to differentiate and form tissues.
“The positive side of this discovery is that now we know cells make these modifications and it impacts their fate,” explains Dr Gentleman. “When we provide stem cells with a 3D structure to help them form a tissue, we have to remember that they will modify the environment we present to them. To really coax them to form the tissue we want, we have to find ways to harness this effect so that the local environment they create is one that will drive their differentiation down the correct path.”
Dr Holger Auner, a blood cancer specialist and one of the Imperial College London authors on the paper, said: “Engineering tissues to replace damaged or diseased tissue could ultimately help patients with a variety of conditions - including cancer. We hope that this insight will also help us to study how cancer cells interact with the tissue that surrounds them, which may lead to better therapies.”
Notes:
“Bi-directional cell-pericellular matrix interactions direct stem cell fate” isto be published in Nature Communications on 3 October, 10.00 BST. It can be viewed at Nature Communications under the Digital Object Identifier number 10.1038/s41467-018-06183-4 once published. View the paper online here: https://rdcu.be/8oKF.
Dr Eileen Gentleman is a Principal Investigator in the Centre of Craniofacial and Regenerative Biology at King’s College London Dental Institute. The Gentleman lab works at the interface of stem cell biology, chemistry and materials science to develop innovative biomaterials for regenerative medicine.

