Bacteria use an array of mechanisms to segregate their chromosomes, but in spite of years of study, we still understand this phenomenon very poorly at the molecular level. We have resolved a long-standing controversy on the role of the motor protein ParA in this process.”
Alex Parker
27 August 2021
Cryo-electron microscopy study unveils molecular details of bacterial cell division
New research from the Bergeron Lab has profound implications on our fundamental understanding of bacterial cell division.
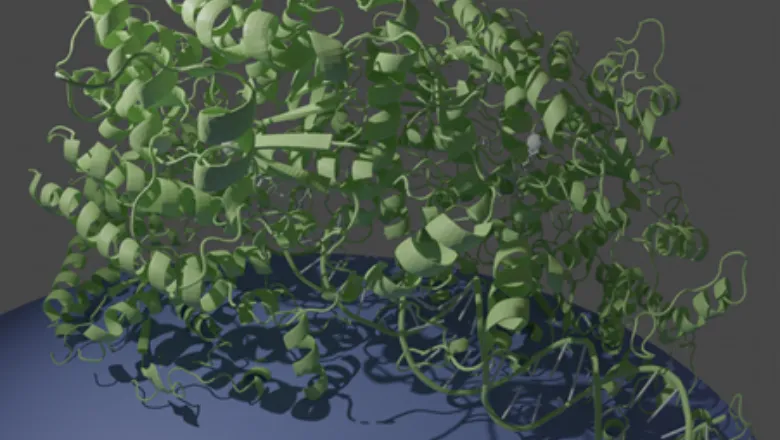
Every cell possesses a specific machinery to ensure that its genetic material gets separated during cell division. In humans and other multicellular organisms this machinery consists of microtubules, that attach to dividing chromosomes and pull them apart.
Scientists know that bacterial cells possess a different, evolutionarily unrelated machinery to achieve the same result, but the molecular details of how this machinery operates is still not fully understood. Most significantly, because it is different from its human counterpart, understanding how this bacterial machinery operates could be exploited for the development of new antibiotics.
In their latest research, published today by Nature Communications, PhD student Alex Parker and Dr Julien Bergeron used one of the world’s most powerful electron microscopes to look at the protein ParA, the “motor” of the bacterial DNA segregation machinery, which uses the energy from ATP to power the motion of DNA across the cell.
With molecular accuracy, they were able to observe that this protein forms a tight filament on DNA, dramatically rearranging its internal molecular structure in the process. It then uses the energy of ATP to jump off the DNA, pulling the newly-synthesized DNA molecule with it. This illustrates that the cell’s own DNA, coated with ParA, act as “rail tracks”, to move the bacterium’s genetic material across the cell as it replicates.
These results could lead to a major paradigm shift in the field. The ParA protein is near-ubiquitous in bacteria, yet its mechanism of action had been highly controversial. This work by Parker and Bergeron conclusively demonstrates the formation of filaments by the ParA protein on DNA, and its coupling with ATP hydrolysis.
Significantly, because the ParA protein is unique to bacteria it could be targeted by a new generation of antibiotics. This study was performed in the human pathogen V. cholerae - the aertiologic agent of cholera - a disease that is endemic in the developing world and one that is resistant to many antibiotics.
The scientists are now turning to ParB, a second protein that acts as an adapter between the ParA filaments and the nascent DNA molecules. They are currently in the process of reconstituting the complex formed with ParA, ParB, and the cargo DNA, to elucidate the molecular details of their interaction.
In addition, they plan to establish if the proposed mechanism for ParA function is universal to the family of motor proteins that use the energy from ATP to localize elements in the bacterial cell, or if (more likely) they use other elements, such as the bacterial membrane, as tracks to promote localization.
Speaking on the significance of their study Dr Julien Bergeron said:
Our work demonstrates that most bacteria use their own DNA as rail-tracks to move newly-synthesized molecules across the cell. Almost 60 years after Rosalind Franklin’s Photo 51, this is a reminder that DNA hasn’t revealed all its secrets yet.”
Dr Julien Bergeron
Read the full paper "The structure of the bacterial DNA segregation ATPase filament reveals the conformational plasticity of ParA upon DNA binding" in Nature Communications.

