20 August 2019
Dual Sympathetic Input into Developing Salivary Glands
Results published in the Journal of Dental Research highlight for the first time the detailed developmental time course of TH-expressing neurons during murine SG development and suggest a role for these neurons in branching morphogenesis.
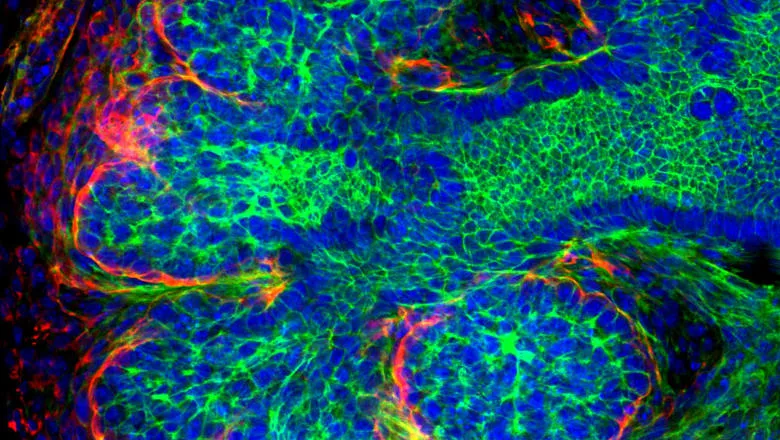
Researchers at King’s College London’s Faculty of Dentistry, Oral & Craniofacial Sciences have published work in the Journal of Dental Research.
Neuronal signalling is known to be required for salivary gland development, with parasympathetic nerves interacting with the surrounding tissues from early stages to maintain a progenitor cell population and control morphogenesis. In contrast, post-ganglionic sympathetic nerves arrive late in salivary gland development to perform a secretory function, however no previous report has shown their role during development.
Here we show that a subset of neuronal cells within the parasympathetic submandibular ganglion (PSG) express the catecholaminergic marker Tyrosine Hydroxylase (TH) in both developing murine and human submandibular glands. This sympathetic phenotype coincided with the expression of transcription factor Hand2 within the PSG from the bud stage (E12.5) of mouse embryonic SG development, Hand2 has been previously associated with the decision of neural crest cells to become sympathetic in other systems suggesting a role in controlling neuronal fate in the salivary gland. These sympathetic-like neurons provide a population of TH expressing neurons prior to the arrival of the post-ganglionic sympathetic axons from the superior cervical ganglion (SCG) at E15.5. In culture, in the absence of nerves from the SCG, these PSG derived TH neurons were clearly evident forming a network around the gland. Chemical ablation of dopamine receptors in explant culture using the neurotoxin 6-Hydroxydopamine (6-OHDA) at early stages of gland development resulted in specific loss of the TH-positive neurons from the PSG and subsequent branching was inhibited.
Taken altogether, these results highlight for the first time the detailed developmental time course of TH-expressing neurons during murine SG development and suggest a role for these neurons in branching morphogenesis.

