We hope our study helps lift the veil of mystery surrounding the mechanisms that control neural stem cell activity in the adult and especially the ageing brain and may allow us to develop new strategies to defeat deadly brain cancer stem cells.”
Professor Benedikt Berninger, the lead author of the study.
21 April 2023
Key protein responsible for controlling production of new neurons for memory and learning in the adult brain identified
Scientists reported the crucial role of transcriptional co-activator Yap1 in regulating adult hippocampal neurogenesis and its potential involvement in brain cancer.
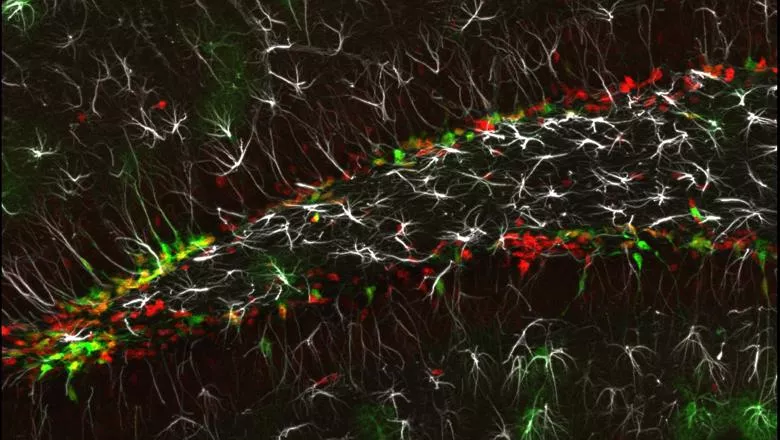
A new publication out today in The EMBO Journal identified a key protein in the molecular mechanism triggering neurogenesis in the hippocampus. They found that tight regulation of Yap1 activity is essential as dysregulation can cause tissue disruption seen in the early stages of brain cancer.
Neurogenesis is the process by which new neurons are produced by neural stem cells (NSCs) in the brain. Neurogenesis is a crucial process in embryo development, but it also continues in some brain regions after birth and all throughout adulthood. In adulthood, neurogenesis is mainly responsible for brain plasticity.
In the adult hippocampus, a brain area responsible for memory and learning, most stem cells are held at quiescence. This reversible pause protects stem cells against damage and controls the rate of neurogenesis. When necessary, the stem cells can be taken off this pause to undergo activation. The mechanisms controlling quiescence and activation are still not fully understood.
The researchers in the Centre for Developmental Neurobiology sought to understand the mechanism underlying neurogenesis in the adult hippocampus. Upon analysing RNA sequencing data, they found that Yap1 is enriched in activated NSCs. This observation prompted an in-depth investigation into the role of Yap1.
They used primary cell culture from adult hippocampal tissue, a proven model to study the transition between NSCs states of quiescence and activation. They confirmed that the transfer of Yap1 from the cytoplasm to the nucleus accompanies NSCs activation, with the reverse occurring as the NSCs return to quiescence.
They then looked for the consequences of abnormal Yap1 protein level in vivo. Even though the short-term effect was small, deleting the Yap1 protein decreased NSCs activation long term. This confirms NSCs activation is influenced by Yap1, with other compensatory mechanisms yet to be identified.
The next step was to observe the consequences of Yap1 overexpression. Interestingly, overexpressing Yap1 does not induce activation, indicating the presence of very tight upstream control. To overrule this control, they overexpressed a mutant Yap1 protein that is resistant to phosphorylation, a type of protein modification. They observed that this does promote activation, indicating that phosphorylation is involved in the upstream control mechanism of Yap1.
The overexpression of this mutant Yap1 protein also induces the expression of other proteins which have been implicated in glioblastoma. This type of brain tumour is known to be fast-growing and very aggressive. In fact, the long-term expression of mutant Yap1 caused massive disruption of brain tissue. This discovery suggests that loss of Yap1 control could be a key step in the initiation of brain tumour.
The authors noted that this discovery warrants further investigation into Yap1 in adult neurogenesis, especially during ageing and brain cancer.
For more information, please contact Annora Thoeng (School of Neuroscience Senior Communications and Engagement Officer)

