I am honoured for such an award to our truly interdisciplinary effort. I think Molecular Simulations and Protein Design are becoming essential instruments in the elucidation of molecular mechanisms that are often inaccessible to direct experimentation. We are fortunate to be part of this exciting moment for Structural and Computational Biology. The successes of Alphafold and Deepmind in the field clearly demonstrate that computation is an essential part of modern Science.”
Professor Franca Fraternali
15 October 2021
King's academics receive National Physics Laboratory's most prestigious award
Study on new pre-clinical therapeutic paradigm for tackling antimicrobial resistance wins the Rayleigh Award for most outstanding published paper.
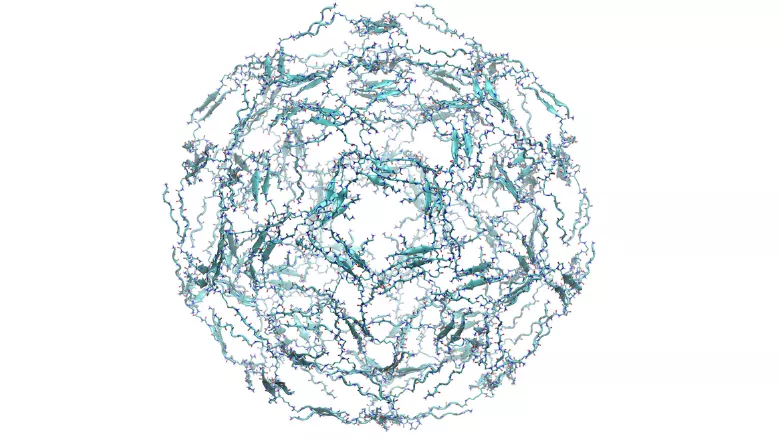
Professors Franca Fraternali and Chris Lorenz, based in the Randall Centre for Cell & Molecular Biophysics and the Department of Physics respectively, have won the annual Rayleigh Award for their paper Engineering chirally blind protein pseudocapsids into antibacterial persisters published in ACS Nano.
The paper contains promising evidence for developing a synthetic antibiotic, specifically designed to fight resistant bacterial infections, and was the result of collaboration between King’s, the National Physical Laboratory, the University of Cambridge, University of Exeter and UCL.
Professor Franca Fraternali commented:
This interdisciplinary work introduces a novel pre-clinical therapeutic paradigm for tackling antimicrobial resistance (AMR) by applying geometric principles to the design of artificial capsids or virus-like particles with specific biological functions. The experimental design is supported by state-of-the-art molecular dynamics to extract new design principles for antimicrobial agents with predictable properties.
The King’s team carried out simulations of the nanoparticles of antimicrobial peptides, which provided: (a) a detailed description of the molecular scale interactions which are key to the formation and stabilisation of the antimicrobial peptide nanoparticles, (b) an understanding of the general structure of the peptides within the nanoparticles and (c) a detailed description of the mechanism of action of the resulting nanoparticles when interacting with model bacterial membranes - all of which provide a perfect complement to the experimental work done in the groups at NPL, University of Cambridge, University of Exeter and UCL.
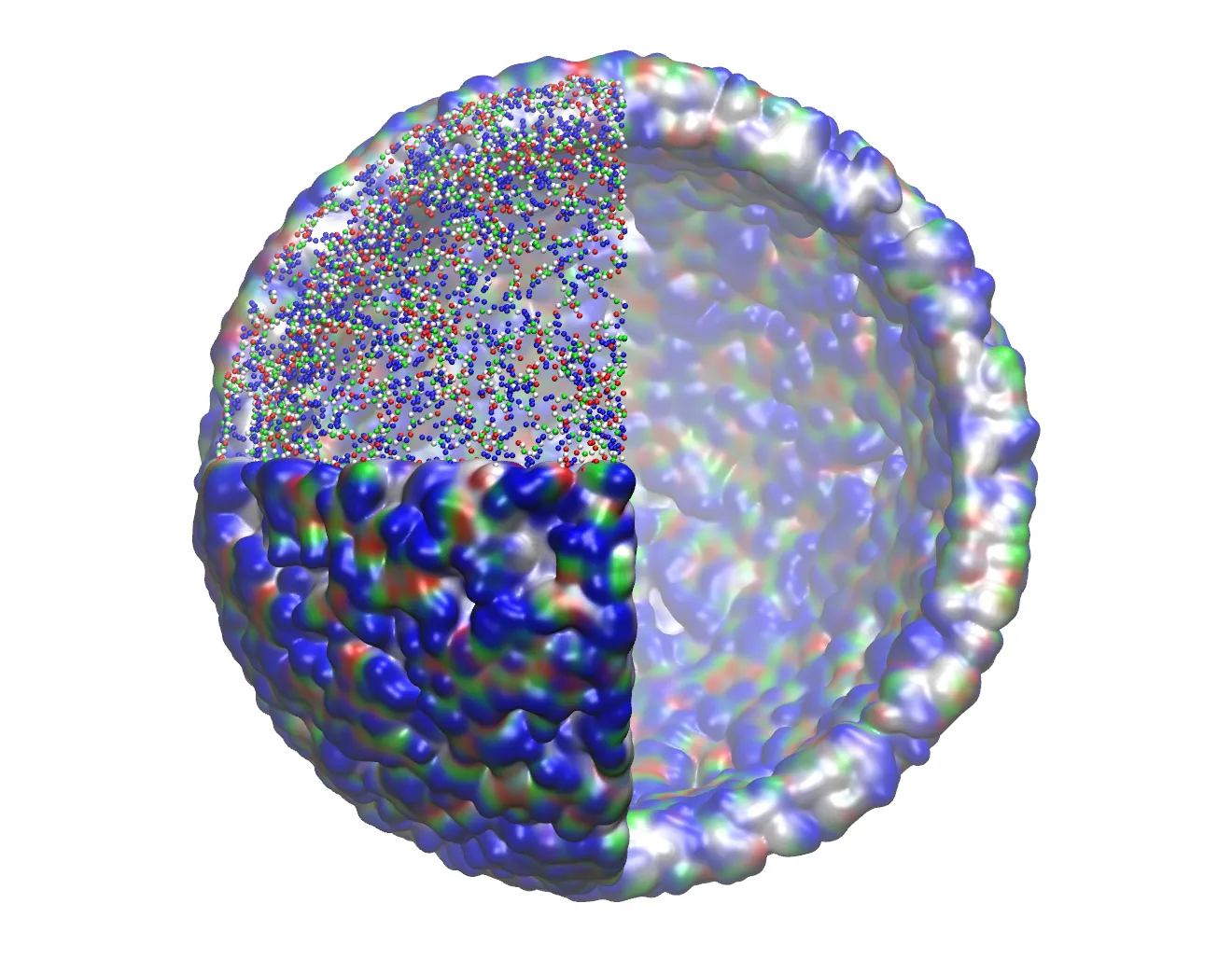
I am very happy that this paper has been recognised by NPL as I think that it is a nice demonstration of the level of understanding that can be gained from an interdisciplinary study that provides insight from across various scales (in this case from molecular to cellular). The computational modelling performed at King’s for this study is a perfect example of how these tools can be used to assist in the design and to provide the molecular-scale understanding of the structure and function of therapeutic nanoparticles which is currently unobtainable from experiment.”
Professor Chris Lorenz
The Rayleigh Award is highly competitive and papers are judged on their creativity and novelty, the extent and quality of the scientific investigation, potential impact, and clarity and accessibility.
The Rayleigh Award is named after John William Strutt Rayleigh, Chairman of the Government Committee that recommended the formation of NPL in 1897.


