Our study is the first to show evidence of this widespread gene regulation mechanism in neurodegenerative diseases. The discovery of this mechanism has the potential to help generate more advanced therapies for a huge variety of human neuropathologies.
Lead researcher EMBO Postdoctoral Research Fellow Dr Patricia Gordon from Centre for Developmental Neurobiology (CDN) at the Institute of Psychiatry, Psychology & Neuroscience (IoPPN) at King’s College London
26 March 2021
Researchers discover new gene mechanism underlying neurodegenerative disorders
Researchers from King’s College London and the Francis Crick Institute have discovered a key gene mechanism which is associated with neurodegenerative disorders.
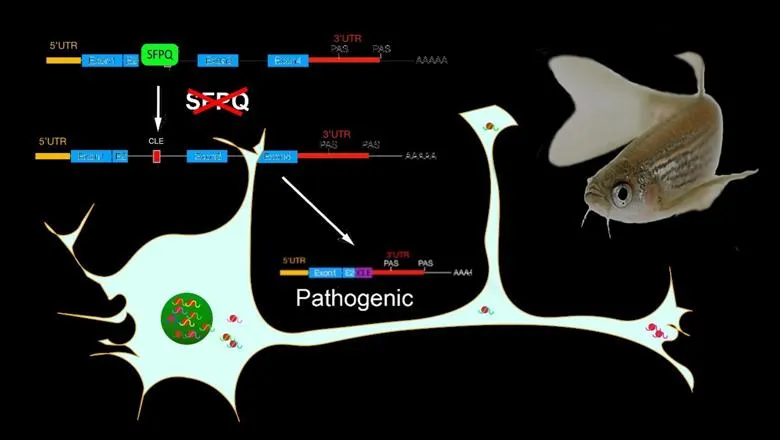
The study, published in Nature Communications today, has revealed a new gene mechanism that explains the association between the protein, SFPQ, and Amyotrophic Lateral Sclerosis (ALS). SFPQ has also been implicated in other neurodegenerative disorders, including Frontotemporal Dementia (FTD) and Alzheimer’s Disease.
SFPQ is a protein which regulates gene expression within cells in the nervous system, known as neurons. Previous research has shown that the SFPQ protein is associated with human neurodegenerative diseases, however, only recently has a mechanism explaining these findings been discovered.
Professor Corinne Houart, Deputy Head at the Centre for Developmental Neurobiology (CDN) at the Institute of Psychiatry, Psychology & Neuroscience (IoPPN), leads the Houart Lab at the Francis Crick Institute, where her research team specialise in understanding the biological mechanisms underlying neurodegeneration.
The finding that losing SFPQ function leads to formation of short abnormal mRNAs able to produce small interfering proteins indicates that neurodegenerative disorders losing SFPQ activity are producing pathogenic short mRNAs. These may be effective targets for therapy.
Professor Corinne Houart, Deputy Head at the Centre for Developmental Neurobiology (CDN) at the IoPPN
Through studying the blinding between SFPQ and various RNA’s in Zebrafish, the researchers have revealed a mechanism explaining the association between the SFPQ protein and neurodegenerative diseases.
The study found that the SFPQ protein protects neuronal genes from forming unusual short RNA sequences, known as CLE transcripts. mRNA isoforms containing CLE transcripts were found to encode one specific peptide which was responsible for some of the nervous system abnormalities caused by a lack of the SFPQ protein.
This paper is the first to show evidence of this widespread gene regulation mechanism in neurodegenerative diseases. The discovery of this mechanism has the potential to help generate more advanced therapies for a huge variety of human neuropathologies.
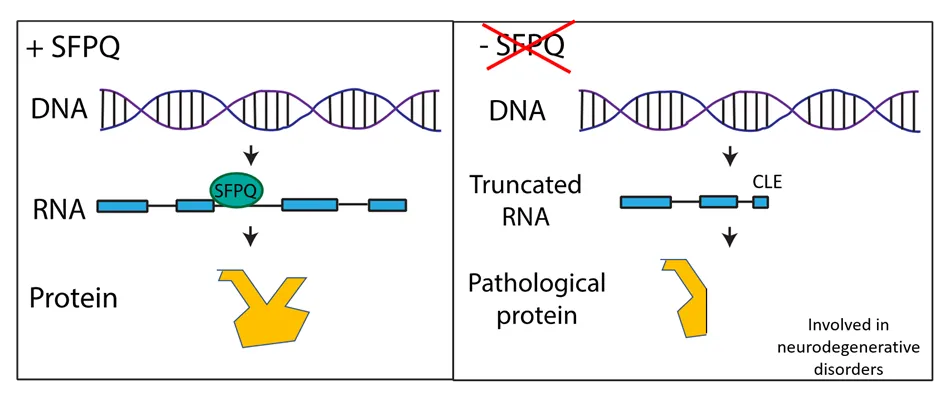
Through studying the mRNAs affected by loss of SFPQ in zebrafish, the researchers have revealed a mechanism explaining the association between the SFPQ protein and neurodegenerative diseases.
The study found that the SFPQ protein protects neuronal genes from forming unusual short RNA sequences, known as CLE transcripts. These CLE transcripts were found to encode short proteins, some responsible for specific nervous system abnormalities caused by lack of the SFPQ protein. CLE transcripts are found in ALS iPSC-derived neurons and patients’ tissues.
This study was funded by the Biotechnology and Biological Sciences Research Council, the European Commission, the Wellcome Trust, and EMBO.
Paper reference: Gordan, P. et al. (2021) “A conserved role for the ALS-linked splicing factor SFPQ in repression of pathogenic cryptic last exons”. Nature Communications. DOI: 10.1038/s41467-021-22098-z. : http://dx.doi.org/
Contact
For further information please contact Louise Pratt, Head of Communications, Institute of Psychiatry, Psychology & Neuroscience, King’s College London
Tel: +44 7850 919020
Email: louise.a.pratt@kcl.ac.uk

