The Biobank
The Haematology Biobank is based at the Rayne Institute, part of the Denmark Hill Campus of King’s College London. Our aim is to support and facilitate basic and translational research into the aetiology, diagnosis and prognosis of blood diseases, through the support and generosity of Haematology patients.
Biobanking Haemato-oncology samples at the Rayne Institute was first established in 2005 with funding from Blood Cancer UK, but we now biobank samples to support a whole range of Haematological research, from blood cancers to red cell and clotting disorders. We work closely with clinical teams, particularly at King’s College Hospital NHS Trust, and have capacity to biobank surplus diagnostic material from regional hospitals, via the South East Haematological Malignancy Diagnostic Centre (SEHMDS). We currently process approximately 1500 samples per year, generating around 8000 aliquots of viable cells and tissue components from bone marrow, blood and lymphoid tissues, for research.
We welcome applications to access samples from researchers across King’s Health Partners organisations and beyond, as well as commercial/ industry researchers working in active academic collaborations.
The Biobank operates under REC approval 23/NE/0160 (Designated Individual (DI) Claire Troakes & HTA Licence Number 12293). Researchers using material from the Biobank must do so within the stipulations of the Material Transfer Agreement and the conditions of our ethical approval.
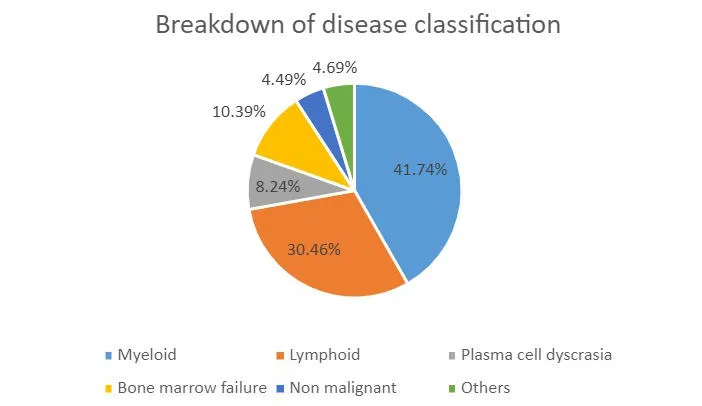
What's in the Biobank
We bank primarily material from bone marrow aspirates and peripheral blood. Collection of other tissue types for specific projects are possible e.g. lymphoid tissues, skin and buccal washes (for constitutional DNA), faeces and urine. Researchers may apply to request specialised biobanking. We also offer biobanking services for clinical trials. We encourage researchers to engage with the Biobank at the earliest opportunity to plan for clinical trial biobanking. We can help with grant applications by providing detailed costs and quotes. Please contact the Biobank Laboratory Manager (Rajani Chelliah) for more information.
These are publications of research made possible with support from the Biobank samples.
- Thakurta, A., A. K. Gandhi, M. F. Waldman, C. Bjorklund, Y. Ning, D. Mendy, P. Schafer, A. Lopez-Girona, S. Lentzsch, S. A. Schey, Y. Calle, R. Chelliah, R. Z. Orlowski, A. Madan, H. Avet-Loiseau and R. Chopra (2014). "Absence of mutations in cereblon (CRBN) and DNA damage-binding protein 1 (DDB1) genes and significance for IMiD therapy." Leukemia 28(5): 1129-1131
- Gaymes TJ, Mohamedali AM, Patterson M, Matto N, Smith A, Kulasekararaj A, Chelliah R, Curtin N, Farzaneh F, Shall S, Mufti GJ (2013). PARP inhibitor sensitivity in high risk MDS and acute myeloid leukaemia is associated with microsatellite instability dependent frameshift mutations in DNA repair genes. Haematologica 98(9):1397-406
- Kordasti, S. Y., B. Afzali, Z. Lim, W. Ingram, J. Hayden, L. Barber, K. Matthews, R. Chelliah, B. Guinn, G. Lombardi, F. Farzaneh and G. J. Mufti (2009). "IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low-risk myelodysplastic syndrome." Br J Haematol 145(1): 64-72.
- Syed A. Mian,1,2 Ander Abarrategi,2 Kar Lok Kong,1 Kevin Rouault-Pierre,2 Henry Wood,1,3 Caroline A. Oedekoven,2 Alexander E. Smith,1,3 Antoniana Batsivari,2 Linda Ariza-McNaughton,2 Peter Johnson,4 Thomas Snoeks,4 Ghulam J. Mufti,1,3,#* and Dominique Bonnet2, Ectopic Humanized Mesenchymal Niche in Mice Enables Robust Engraftment of Myelodysplastic stem Cells (Blood Cancer Discov. 2021 Mar; 2(2): 135–145)
- Shok Ping Lim1, Benedetta Costantini 1 2, Syed A Mian 1 3, Pilar Perez Abellan 1 4, Shreyans Gandhi 4, Marc Martinez Llordella 5, Juan Jose Lozano 6, Rita Antunes Dos Reis 7, Giovanni A M Povoleri 5, Thanos P Mourikis 8, Ander Abarrategi 3, Linda Ariza-McNaughton 3, Susanne Heck 9, Jonathan M Irish 10, Giovanna Lombardi 11, Judith C W Marsh 1 4, Dominique Bonnet 3, Shahram Kordasti 7 12, Ghulam J Mufti 1 4 Treg sensitivity to FasL and relative IL-2 deprivation drive idiopathic aplastic anemia immune dysfunction ( 2020 Aug 13; 136(7): 885–897)
- K Rouault-Pierre,1,7S A Mian,1,2,7 M Goulard,3 A Abarrategi,1 A Di Tulio,1 A E Smith,2,4 A Mohamedali,2 S Best,2 A-M Nloga,5 A G Kulasekararaj,4 L Ades,5 C Chomienne,3,6 P Fenaux,3,5 C Dosquet,3,6,8,* G J Mufti,2,4,8,* and D Bonnet1,8,* Preclinical modelling of myelodysplastic syndromes (Leukemia. 2017 Dec; 31(12): 2702–2708)
- Sneha Chitre1, Friedrich Stölzel 2, Kirsty Cuthill 3, Mathew Streetly 3, Charlotte Graham 3, Claudia Dill 2, Azim Mohamedali 3, Alexander Smith 3, Johannes Schetelig 2 4, Heidi Altmann 2, Martin Bornhäuser 3 2, Ghulam J Mufti Clonal hematopoiesis in patients with multiple myeloma undergoing autologous stem cell transplantation Leukemia volume 32, pages2020–2024 (2018)
- Alexander E Smith1, Austin G Kulasekararaj 1, Jie Jiang 1, Syed Mian 2, Azim Mohamedali 1, Joop Gaken 2, Robin Ireland 3, Barbara Czepulkowski 3, Steven Best 3, Ghulam J Mufti 4 (CSNK1A1 mutations and isolated del(5q) abnormality in myelodysplastic syndrome: a retrospective mutational analysis Lancet Haematol 2015 May;2(5):e212-21
- Austin G. Kulasekararaj,1,2Jie Jiang,1,2 Alexander E. Smith,1,2 Azim M. Mohamedali,1,2 Syed Mian,1 Shreyans Gandhi,2 Joop Gaken,1 Barbara Czepulkowski,2 Judith C. W. Marsh,1,2 and Ghulam J. Mufti1,2 Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome ( 2014 Oct 23; 124(17): 2698–2704)
- Syed A. Mian,1,*Kevin Rouault-Pierre,2,* Alexander E. Smith,1,3 Thomas Seidl,1 Irene Pizzitola,2 Aytug Kizilors,3 Austin G. Kulasekararaj,1,3 Dominique Bonnet,a,2 and Ghulam J. Muftib,1,3 SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment (Nat Commun. 2015; 6: 10004)
- Austin G. Kulasekararaj, Alexander E. Smith, Syed A. Mian, Azim M. Mohamedali, Pramila Krishnamurthy, Nicholas C. Lea, Joop Gäken, Coralie Pennaneach, Robin Ireland, Barbara Czepulkowski, Sabine Pomplun, Judith C. Marsh, Ghulam J. Mufti TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5 and correlate with adverse prognosis. (Br J Haematol 2013)
- Syed A. Mian,1,*Alexander E. Smith,1,* Austin G. Kulasekararaj,1,2 Aytug Kizilors,2 Azim M. Mohamedali,1 Nicholas C. Lea,2 Konstantinos Mitsopoulos,3 Kevin Ford,1 Erick Nasser,1 Thomas Seidl,1 and Ghulam J. Mufti1,2 Spliceosome mutations exhibit specific associations with epigenetic modifiers and proto-oncogenes mutated in myelodysplastic syndrome ( 2013 Jul; 98(7): 1058–1066)
The Biobank Governance Committee oversees Biobanking activity to ensure compliance with HTA rules and the conditions of our ethical approval. It is aided by an Operations Subcommittee. Applications for access to research samples are considered by an Access Subcommittee which meets quarterly.
Biological Service
Access
Governance – Dr Lynn Quek( Lynn.quek@kcl.ac.uk ) and Dr Piers Patten piers.patten@kcl.ac.uk
Biobank laboratory aspects quotes and clinical trial projects queries – Rajani.chelliah@kcl.ac.uk
Applications for access to research samples – Asmaa.Elshahawy@kcl.ac.uk
Applications for access to research samples & other useful documents for Researchers
We welcome applications from researchers to access our samples. This is done via a formal process that involves a review by the Biobank Access Subcommittee. For additional information, please contact Asmaa Elshahawy.
Applications for Sample Access
Researchers seeking access to our samples must first refer to our sample access policy to ensure that they are eligible to apply. Our bank’s inventory is not presently available for external scrutiny: researchers must contact the data Manager who will conduct searches as required. Applications to receive material must be made on a pro forma supplied by the Biobank Manager. All applications are assessed by our Biobank Management Committee, comprising an independent academic reviewer, senior departmental clinical and non-clinical academics, a lay representative / donor advocate and the Designated Individual and Tissue Bank Manager.
Our Sample Access Policy
The Tissue Bank will make samples available to bona fide researchers, wherever their research is conducted. We consider bona fide researchers to be those directed or supervised by a recognised public, charitable or industrial body. Where we are unsure of a researcher’s bona fides (for example, where requests are from smaller industrial or private bodies) we will look for a track record in the field or other unambiguous means of validating their credentials in making our decision.
Any use of samples must be fully consistent with the consent given by our donors.
Any use of samples must be fully consistent with the generic ethics approval described under NE/18/0141. Where a project falls outside of our generic ethics approval, we will require applicant to secure project-specific approval from an appropriate Research Ethics Committee before releasing material to them.
Applicants must satisfy us that the stated research presents no undue risks to the donors of the samples.
We will only supply samples for research that has been appropriately reviewed and validated. We expect that most applications will be supported by funders who carry out peer review of scientific merit or that applicants will be able to provide evidence of other review. Where we are not satisfied that a study has been adequately reviewed, we reserve the right to commission a review on our behalf.
Patient Information Sheet and Consent Form
The current versions of our Patient Invitation Letter, Patient Information Sheet, and Consent Form are available for printing using the links above.
These are version-controlled documents which are updated from time to time. The latest versions will be available via this website. Please do not store local copies and ensure that the latest version of documents is used.
What's in the Biobank
We bank primarily material from bone marrow aspirates and peripheral blood. Collection of other tissue types for specific projects are possible e.g. lymphoid tissues, skin and buccal washes (for constitutional DNA), faeces and urine. Researchers may apply to request specialised biobanking. We also offer biobanking services for clinical trials. We encourage researchers to engage with the Biobank at the earliest opportunity to plan for clinical trial biobanking. We can help with grant applications by providing detailed costs and quotes. Please contact the Biobank Laboratory Manager (Rajani Chelliah) for more information.

Facility staff
These are publications of research made possible with support from the Biobank samples.
- Thakurta, A., A. K. Gandhi, M. F. Waldman, C. Bjorklund, Y. Ning, D. Mendy, P. Schafer, A. Lopez-Girona, S. Lentzsch, S. A. Schey, Y. Calle, R. Chelliah, R. Z. Orlowski, A. Madan, H. Avet-Loiseau and R. Chopra (2014). "Absence of mutations in cereblon (CRBN) and DNA damage-binding protein 1 (DDB1) genes and significance for IMiD therapy." Leukemia 28(5): 1129-1131
- Gaymes TJ, Mohamedali AM, Patterson M, Matto N, Smith A, Kulasekararaj A, Chelliah R, Curtin N, Farzaneh F, Shall S, Mufti GJ (2013). PARP inhibitor sensitivity in high risk MDS and acute myeloid leukaemia is associated with microsatellite instability dependent frameshift mutations in DNA repair genes. Haematologica 98(9):1397-406
- Kordasti, S. Y., B. Afzali, Z. Lim, W. Ingram, J. Hayden, L. Barber, K. Matthews, R. Chelliah, B. Guinn, G. Lombardi, F. Farzaneh and G. J. Mufti (2009). "IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low-risk myelodysplastic syndrome." Br J Haematol 145(1): 64-72.
- Syed A. Mian,1,2 Ander Abarrategi,2 Kar Lok Kong,1 Kevin Rouault-Pierre,2 Henry Wood,1,3 Caroline A. Oedekoven,2 Alexander E. Smith,1,3 Antoniana Batsivari,2 Linda Ariza-McNaughton,2 Peter Johnson,4 Thomas Snoeks,4 Ghulam J. Mufti,1,3,#* and Dominique Bonnet2, Ectopic Humanized Mesenchymal Niche in Mice Enables Robust Engraftment of Myelodysplastic stem Cells (Blood Cancer Discov. 2021 Mar; 2(2): 135–145)
- Shok Ping Lim1, Benedetta Costantini 1 2, Syed A Mian 1 3, Pilar Perez Abellan 1 4, Shreyans Gandhi 4, Marc Martinez Llordella 5, Juan Jose Lozano 6, Rita Antunes Dos Reis 7, Giovanni A M Povoleri 5, Thanos P Mourikis 8, Ander Abarrategi 3, Linda Ariza-McNaughton 3, Susanne Heck 9, Jonathan M Irish 10, Giovanna Lombardi 11, Judith C W Marsh 1 4, Dominique Bonnet 3, Shahram Kordasti 7 12, Ghulam J Mufti 1 4 Treg sensitivity to FasL and relative IL-2 deprivation drive idiopathic aplastic anemia immune dysfunction ( 2020 Aug 13; 136(7): 885–897)
- K Rouault-Pierre,1,7S A Mian,1,2,7 M Goulard,3 A Abarrategi,1 A Di Tulio,1 A E Smith,2,4 A Mohamedali,2 S Best,2 A-M Nloga,5 A G Kulasekararaj,4 L Ades,5 C Chomienne,3,6 P Fenaux,3,5 C Dosquet,3,6,8,* G J Mufti,2,4,8,* and D Bonnet1,8,* Preclinical modelling of myelodysplastic syndromes (Leukemia. 2017 Dec; 31(12): 2702–2708)
- Sneha Chitre1, Friedrich Stölzel 2, Kirsty Cuthill 3, Mathew Streetly 3, Charlotte Graham 3, Claudia Dill 2, Azim Mohamedali 3, Alexander Smith 3, Johannes Schetelig 2 4, Heidi Altmann 2, Martin Bornhäuser 3 2, Ghulam J Mufti Clonal hematopoiesis in patients with multiple myeloma undergoing autologous stem cell transplantation Leukemia volume 32, pages2020–2024 (2018)
- Alexander E Smith1, Austin G Kulasekararaj 1, Jie Jiang 1, Syed Mian 2, Azim Mohamedali 1, Joop Gaken 2, Robin Ireland 3, Barbara Czepulkowski 3, Steven Best 3, Ghulam J Mufti 4 (CSNK1A1 mutations and isolated del(5q) abnormality in myelodysplastic syndrome: a retrospective mutational analysis Lancet Haematol 2015 May;2(5):e212-21
- Austin G. Kulasekararaj,1,2Jie Jiang,1,2 Alexander E. Smith,1,2 Azim M. Mohamedali,1,2 Syed Mian,1 Shreyans Gandhi,2 Joop Gaken,1 Barbara Czepulkowski,2 Judith C. W. Marsh,1,2 and Ghulam J. Mufti1,2 Somatic mutations identify a subgroup of aplastic anemia patients who progress to myelodysplastic syndrome ( 2014 Oct 23; 124(17): 2698–2704)
- Syed A. Mian,1,*Kevin Rouault-Pierre,2,* Alexander E. Smith,1,3 Thomas Seidl,1 Irene Pizzitola,2 Aytug Kizilors,3 Austin G. Kulasekararaj,1,3 Dominique Bonnet,a,2 and Ghulam J. Muftib,1,3 SF3B1 mutant MDS-initiating cells may arise from the haematopoietic stem cell compartment (Nat Commun. 2015; 6: 10004)
- Austin G. Kulasekararaj, Alexander E. Smith, Syed A. Mian, Azim M. Mohamedali, Pramila Krishnamurthy, Nicholas C. Lea, Joop Gäken, Coralie Pennaneach, Robin Ireland, Barbara Czepulkowski, Sabine Pomplun, Judith C. Marsh, Ghulam J. Mufti TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5 and correlate with adverse prognosis. (Br J Haematol 2013)
- Syed A. Mian,1,*Alexander E. Smith,1,* Austin G. Kulasekararaj,1,2 Aytug Kizilors,2 Azim M. Mohamedali,1 Nicholas C. Lea,2 Konstantinos Mitsopoulos,3 Kevin Ford,1 Erick Nasser,1 Thomas Seidl,1 and Ghulam J. Mufti1,2 Spliceosome mutations exhibit specific associations with epigenetic modifiers and proto-oncogenes mutated in myelodysplastic syndrome ( 2013 Jul; 98(7): 1058–1066)
The Biobank Governance Committee oversees Biobanking activity to ensure compliance with HTA rules and the conditions of our ethical approval. It is aided by an Operations Subcommittee. Applications for access to research samples are considered by an Access Subcommittee which meets quarterly.
Biological Service
Access
Governance – Dr Lynn Quek( Lynn.quek@kcl.ac.uk ) and Dr Piers Patten piers.patten@kcl.ac.uk
Biobank laboratory aspects quotes and clinical trial projects queries – Rajani.chelliah@kcl.ac.uk
Applications for access to research samples – Asmaa.Elshahawy@kcl.ac.uk
Applications for access to research samples & other useful documents for Researchers
We welcome applications from researchers to access our samples. This is done via a formal process that involves a review by the Biobank Access Subcommittee. For additional information, please contact Asmaa Elshahawy.
Applications for Sample Access
Researchers seeking access to our samples must first refer to our sample access policy to ensure that they are eligible to apply. Our bank’s inventory is not presently available for external scrutiny: researchers must contact the data Manager who will conduct searches as required. Applications to receive material must be made on a pro forma supplied by the Biobank Manager. All applications are assessed by our Biobank Management Committee, comprising an independent academic reviewer, senior departmental clinical and non-clinical academics, a lay representative / donor advocate and the Designated Individual and Tissue Bank Manager.
Our Sample Access Policy
The Tissue Bank will make samples available to bona fide researchers, wherever their research is conducted. We consider bona fide researchers to be those directed or supervised by a recognised public, charitable or industrial body. Where we are unsure of a researcher’s bona fides (for example, where requests are from smaller industrial or private bodies) we will look for a track record in the field or other unambiguous means of validating their credentials in making our decision.
Any use of samples must be fully consistent with the consent given by our donors.
Any use of samples must be fully consistent with the generic ethics approval described under NE/18/0141. Where a project falls outside of our generic ethics approval, we will require applicant to secure project-specific approval from an appropriate Research Ethics Committee before releasing material to them.
Applicants must satisfy us that the stated research presents no undue risks to the donors of the samples.
We will only supply samples for research that has been appropriately reviewed and validated. We expect that most applications will be supported by funders who carry out peer review of scientific merit or that applicants will be able to provide evidence of other review. Where we are not satisfied that a study has been adequately reviewed, we reserve the right to commission a review on our behalf.
Patient Information Sheet and Consent Form
The current versions of our Patient Invitation Letter, Patient Information Sheet, and Consent Form are available for printing using the links above.
These are version-controlled documents which are updated from time to time. The latest versions will be available via this website. Please do not store local copies and ensure that the latest version of documents is used.
Contact us
Please contact the Biobank Laboratory Manager (Rajani Chelliah) for more information. KCL Haematology Biobank King's College London Rayne Institute, 123 Coldharbour Lane, London SE5 9NU




