Discovery of pathway leading to depression reveals new drug targets
Scientists have identified the key molecular pathway leading to depression, revealing potential new targets for drug discovery, according to research led by King’s College London’s Institute of Psychiatry. The study, published today in Neuropsychopharmacology, reveals for the first time that the ‘Hedgehog pathway’ regulates how stress hormones, usually elevated during depression, reduce the number of brain cells.
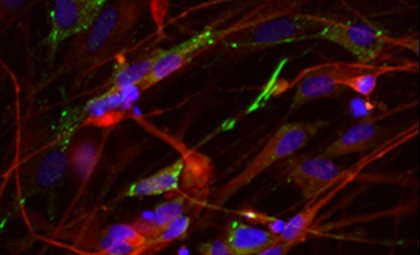
Newborn brain cells (red) and their cell nuclei (blue) as well as synaptic markers (green), which are needed for mature cells to communicate with each other (Image credit: Dr Christoph Anacker).
Depression affects approximately 1 in 5 people in the UK at some point in their lives. The severity of symptoms can range from feelings of sadness and hopelessness to, in the most severe cases, self-harm or suicide. Treatment for depression involves either medication or talking treatment, or usually a combination of the two.
Recent studies have demonstrated that depression is associated with a reduction in a brain process called ‘neurogenesis’- the ability of the brain to produce new brain cells. However, the pathway responsible for this process has, until now, remained unknown.
In this study, Dr Christoph Anacker from the Centre for the Cellular Basis of Behaviour (CCBB) at King’s Institute of Psychiatry and his team studied human stem cells, which are the source of new cells in the human brain, to investigate the effect of stress hormones on brain cell development. The study was funded by the National Institute for Health Research Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and King’s College London and the Medical Research Council UK.
Stress hormones, such as cortisol, are generally elevated in stress and depression. The team studied stem cells in a laboratory and found that high concentrations of cortisol damaged these stem cells and reduced the number of newborn brain cells. They discovered that a specific signalling mechanism in the cell, the ‘Hedgehog pathway’, is responsible for this process. Then, using an animal model, the team confirmed that exposure to stress inhibited this pathway in the brain.
Finally, in order to test the findings, the researchers used a compound called purmorphamine, which is known to stimulate the Hedgehog pathway. They found that by using this drug, they were able to reverse the damaging effects of stress hormones, and normalise the production of new brain cells.
Dr Christoph Anacker, lead author of the study from King’s Institute of Psychiatry says: “By decreasing the number of new-born cells in the human brain, stress hormones damage many important brain functions and may contribute to the development of depression after a period of chronic stress. By inhibiting the Hedgehog signalling pathway, stress hormones reduce the development of immature ‘stem’ cells into mature ‘brain’ cells.”
Dr. Anacker continues: “With as much as half of all depressed patients failing to improve with currently available treatments, developing new, more effective antidepressants still remains a great challenge, which makes it crucial to identify new potential mechanisms to target. The discovery of antidepressants has so far been mainly by serendipity. Developing a drug with a defined effect on the brain, such as increasing the number of new-born brain cells, and with a clear target, such as Hedgehog signalling, will allow us to develop much more specific antidepressants in the future.”
Paper reference: Anacker, C. et al. ‘Glucocoticoid-related molecular signalling pathways regulating hippocampal neurogenesis’ Neuropsychopharmacology (2012) doi: 10.1038/npp.2012.253
Funding: The study was funded by the National Institute for Health Research Biomedical Research Centre for Mental Health at the South London and Maudsley NHS Foundation Trust and King’s College London and the Medical Research Council UK with additional funding from a Commission of European Communities 7th Framework Programme Collaborative Project Grant, a NARSAD Young Investigator Award, Research Councils UK, Italian Ministry of Health, Regione Lombardia and the Italian Ministry of Health.
For further information or interviews with the author, please contact Seil Collins, Press Officer, King’s College London, Institute of Psychiatry. Email: seil.collins@kcl.ac.uk or tel: 0207 848 5377