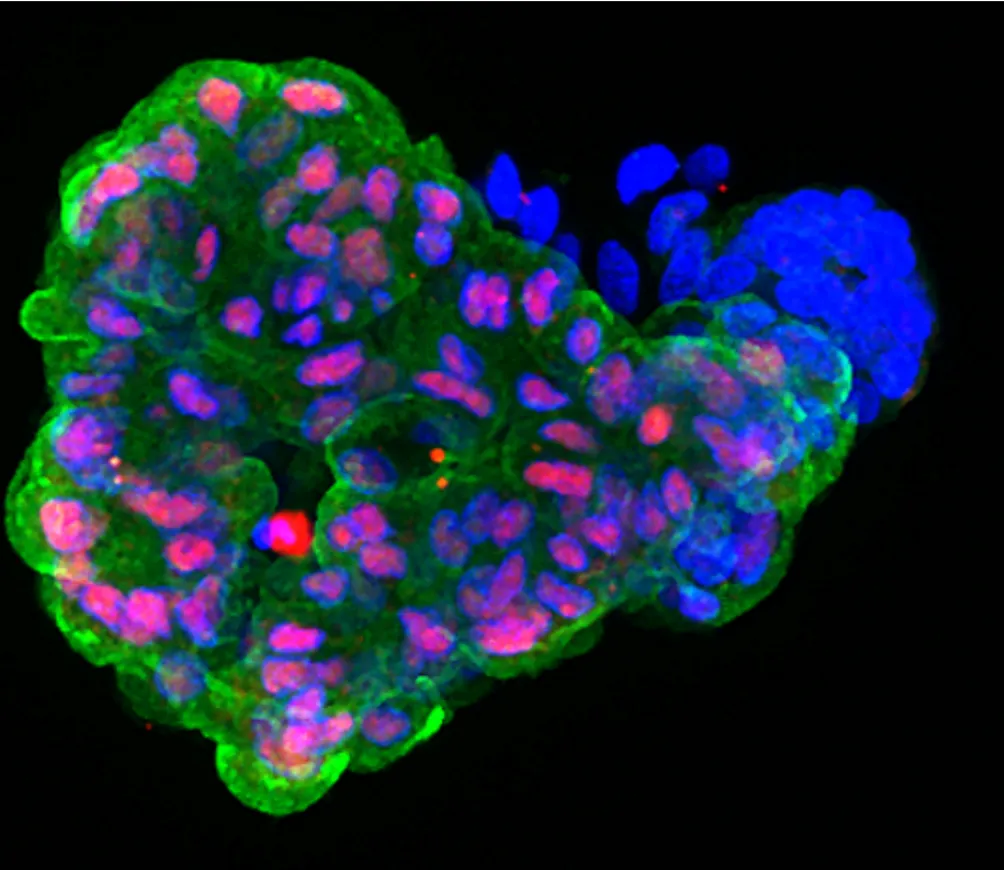
 Early pancreatic organoid made from iPSCs. Red and blue are pancreas proteins and blue shows the nucleus of the cells. Credit: Ana-Maria Cujba
Early pancreatic organoid made from iPSCs. Red and blue are pancreas proteins and blue shows the nucleus of the cells. Credit: Ana-Maria Cujba
For example, in one of our projects we used iPSCs from patients with MODY or which have been genetically modified to have a mutation in HNF1A to generate early pancreas cells which we then culture to make pancreatic organoids. This allowed us to study what goes wrong in the pancreas in patients with MODY in a dish.
As mentioned above, one key avenue of research in our lab focusses on understanding how the cells in the pancreas develop. During the development of the pancreas, there are a number of genes which must be turned on to allow early pancreas cells to become the different cell types in the islet. The role of these genes is well established in determining a cells’ fate, but very little is understood about how they are modified after they become proteins, which can affect how they function and how long they function. For example, when a protein is no longer needed, it can be modified to be destroyed by the cell. We are exploring how this kind of modification affects the proteins needed for producing β cells, with the idea that we could harness the mechanisms that control protein destruction and boost the production of more β cells in a dish.
Another factor that affects beta cell production are the physical properties around them, which of course are difficult to reproduce in a dish even when those cells are grown in 3D. Therefore, we are also exploring different gels to culture organoids in which could help them grow and differentiate into β cells more efficiently.
β cells grown in a dish can be transplanted into patient’s with T1D to replace those destroyed, therefore all the improvements in the differentiation protocols to produce more numbers and more functional beta cells would result in a great advancement in the field.
Single Cell RNA-sequencing
We have already discussed how we use pancreatic organoids to explore our questions. Another of the tools we commonly employ to understand cells is called single cell RNA sequencing (scRNA-seq).
Most of your cells contain exactly the same genome, which is “written” in your DNA. The specialised structure and function of cells is determined by which combinations of genes in your DNA are turned ON (expressed) and OFF (supressed) at different times.