Clinical Trials
Through our clinical trials program, we aim to develop and validate a variety of analytical tools.
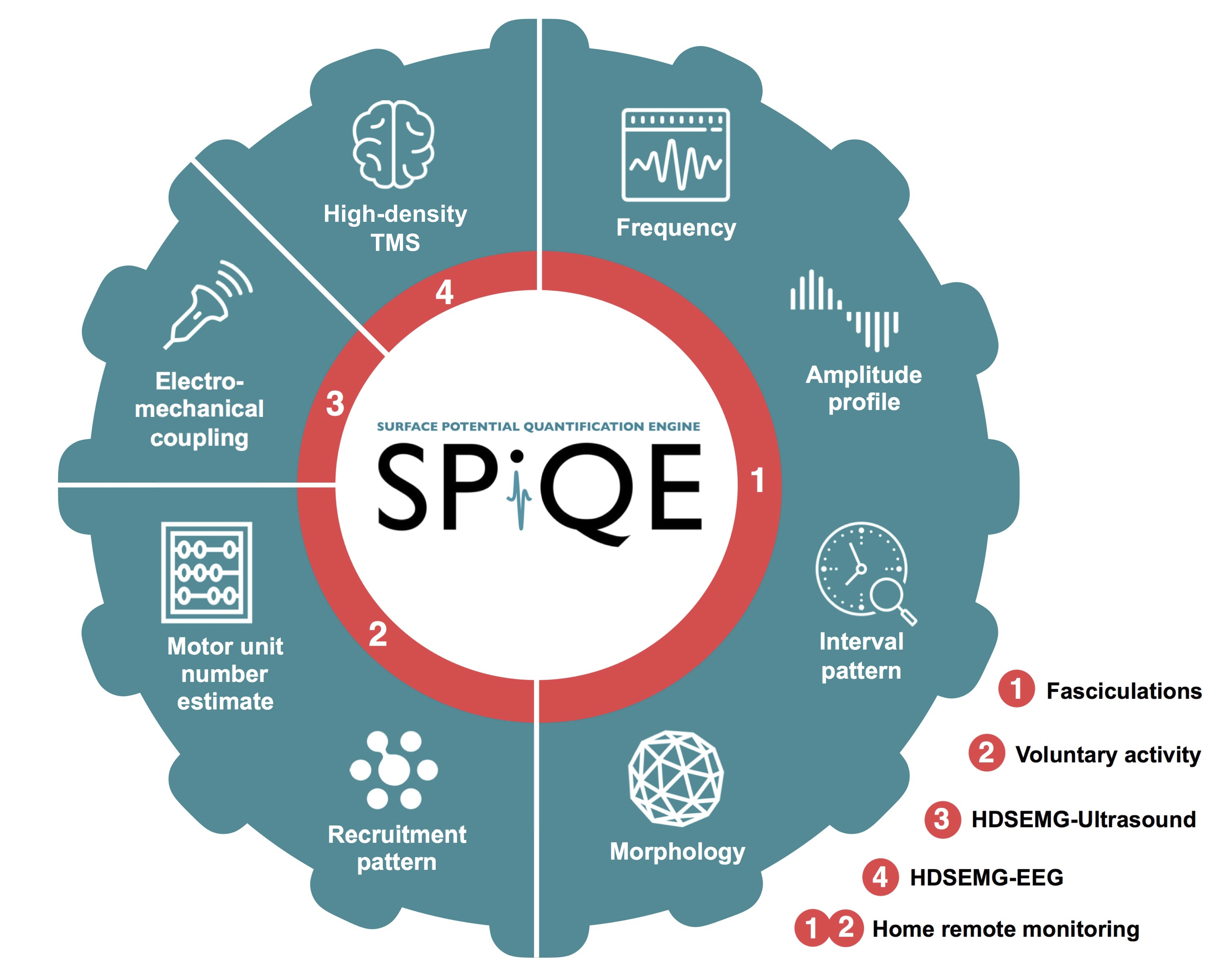
By employing these tools in different research settings, we hope to understand the mechanism and natural history of disease, as we strive to develop and test new biomarkers and therapies.
Clinical trials involve several key components
-
1
Generating a hypothesis: Clinical trials exist in order to test a hypothesis. This is driven by a set of key research questions that are yet to be answered.
-
2
Optimising the study protocol: Deciding upon a suitable and practical study design is an important process. It acts as a guide for all researchers involved in the study. There needs to be careful consideration regarding patient recruitment and the study timeline.
-
3
Recruiting patients: After ethical approval, patients can be screened for the study eligibility criteria. Patients are fully informed about the study protocol, before formal consent is obtained.
-
4
Collecting data: Data are collected and stored according to the study protocol. A unique study ID for each patient ensures anonymisation of patients.
-
5
Performing the analysis: The analytical methods must be validated to ensure accuracy and consistency. Statistical tests are applied to help interpret the findings.
-
6
Publicising the results: Just as important as the study itself is how the results are publicised. This includes publication in appropriate medical journals and presentation at international conferences as well as informing patients and the wider public.
Our current clinical trials
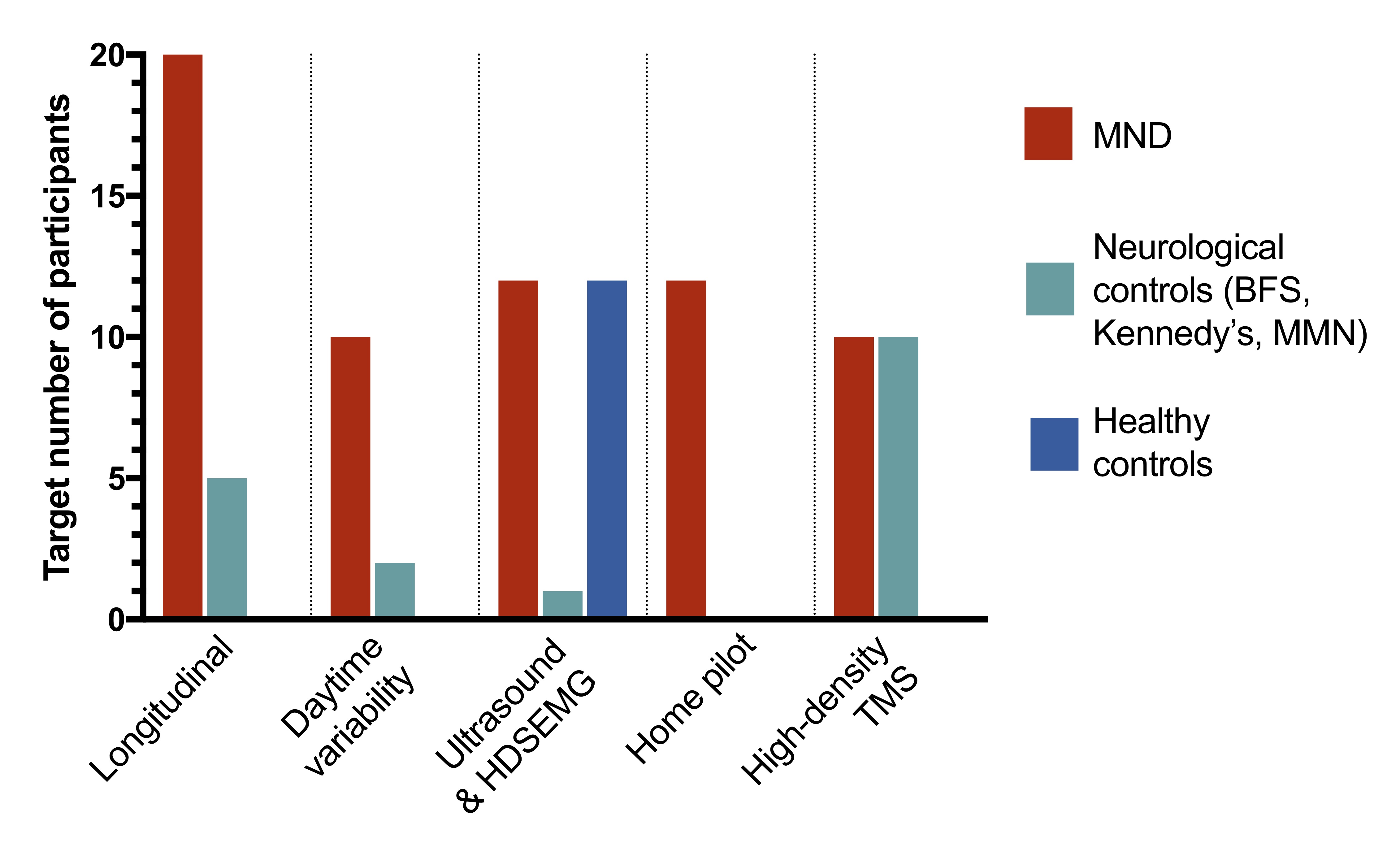
We have several studies that are currently active. The longitudinal fasciculation study collected data every two months for a whole year. The daytime variability study assessed each patient on three occasions throughout a single day (9am, 12pm and 3pm). The ultrasound and EMG study combined these two non-invasive techniques simultaneously to learn about the electromechanical properties of fasciculations.
A more recent project focuses on the introduction of remote home SPiQE monitoring. We believe this approach will dramatically increase the accessibility of this technique, maximising our understanding of neuronal impairment in ALS. As of January 2022, we are excited to launch the recruitment phase for our high-density TMS study.
The target number of patients for each study are shown below (MND=motor neuron disease; BFS= benign fasciculation syndrome; MMN=multifocal motor neuropathy; HDSEMG=high-density surface electromyography; TMS=transcranial magnetic stimulation).
Study progress
The proportion of target data collected for each study is highlighted below. However, even when all data have been collected, there can be a long period of time required for sufficient analysis and dissemination of results.
- Longitudinal: 100%
- Daytime variability: 100%
- Ultrasound & HDSEMG: 100%
- Home pilot: 90%
- High-density TMS: 0%
Please refer to our Publications page for updates on our key results.
If you or someone you know would like to participate in any of our studies, please email us at the address below.
spiqe-info@kcl.ac.uk